
The structure of $ cis-3-hexene $ is:
(A)
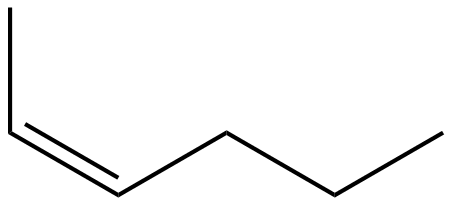
(B)
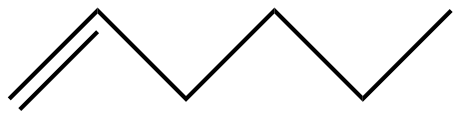
(C)

(D)
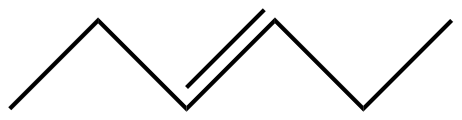




Answer
524.7k+ views
Hint : $ Hex-3-ene $ or $ 3-Hexene $ is a hydrocarbon chain containing six carbon, which are connected by five single bonds, with the exception of the third bond which is a double bond. The third bond is present between the third and the fourth carbon. In the $ cis\; $ isomer both the identical groups attached to the double bonded carbons should be on the same side.
Complete Step By Step Answer:
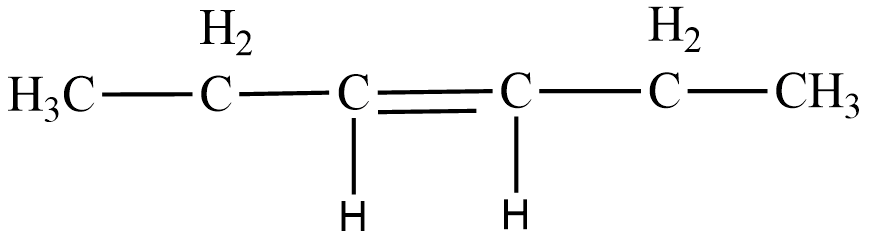
Let us understand the structure of $ 3-Hexene $ to begin with. $ \;Hex- $ means six , hence the chain contains six carbons. To connect these six carbons, we need five single bonds. In $ 3-Hexene $ , the third bond that connects the third and fourth carbon is replaced by a double bond. Thus the structural formula of $ 3-Hexene $ is
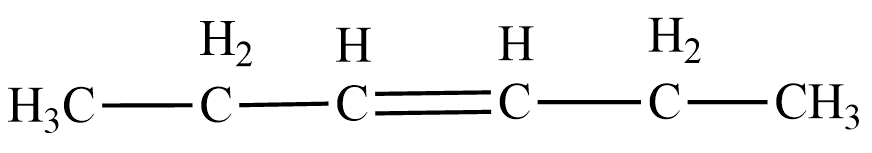
Isomers of a compound have the same chemical formula but different arrangement due to which the properties of the compound also differ.
$ cis- $ and $ trans- $ isomers are the geometrical isomers of a compound. In $ cis- $ isomer, the functional groups attached to the double bonded carbon are on the same side, while in $ trans- $ isomer they are on the opposite sides.
To understand the isomers of $ 3-hexene $ , let us show the structural formula as
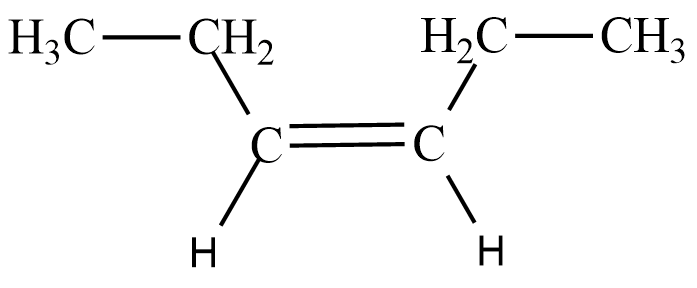
Here, the functional group attached to the double bond carbon is the ethyl group $ C{{H}_{3}}C{{H}_{2}}- $
To obtain the $ cis-3-hexene $ , both functional groups should be on the same side of the double bonded carbon as shown below;
Now, in a skeletal structural formula, only bonds are shown to make the structure simpler. Also, we have to remember that only $ C-C $ bonds are shown in the skeletal structural formula as line segments (without considering the angle between the bonds) and the presence of carbon is shown at the intersection of these line segments. We are not concerned with the number or the position of the $ C-H $ bonds in the skeletal structure.
Keeping these points in mind the skeletal formula for $ cis-3-Hexene $ is shown as

As the angle between the bonds is not important in the skeletal formula, the above formula can be represented as the formula shown in the options
Hence, the correct answer is Option $ (C) $ .
Note :
In the skeletal structure of a hydrocarbon, if at the end of the chain no group is mentioned, we have to always consider a methyl group at the end. In the skeletal structure, the lines show the bonds and the points of intersection of these straight lines or the nodes where the line changes direction shows the presence of carbon, and the hydrogen are balanced as per the remaining valency.
Complete Step By Step Answer:
Let us understand the structure of $ 3-Hexene $ to begin with. $ \;Hex- $ means six , hence the chain contains six carbons. To connect these six carbons, we need five single bonds. In $ 3-Hexene $ , the third bond that connects the third and fourth carbon is replaced by a double bond. Thus the structural formula of $ 3-Hexene $ is
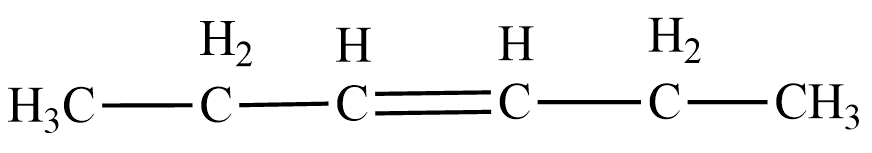
Isomers of a compound have the same chemical formula but different arrangement due to which the properties of the compound also differ.
$ cis- $ and $ trans- $ isomers are the geometrical isomers of a compound. In $ cis- $ isomer, the functional groups attached to the double bonded carbon are on the same side, while in $ trans- $ isomer they are on the opposite sides.
To understand the isomers of $ 3-hexene $ , let us show the structural formula as

Here, the functional group attached to the double bond carbon is the ethyl group $ C{{H}_{3}}C{{H}_{2}}- $
To obtain the $ cis-3-hexene $ , both functional groups should be on the same side of the double bonded carbon as shown below;

Now, in a skeletal structural formula, only bonds are shown to make the structure simpler. Also, we have to remember that only $ C-C $ bonds are shown in the skeletal structural formula as line segments (without considering the angle between the bonds) and the presence of carbon is shown at the intersection of these line segments. We are not concerned with the number or the position of the $ C-H $ bonds in the skeletal structure.
Keeping these points in mind the skeletal formula for $ cis-3-Hexene $ is shown as

As the angle between the bonds is not important in the skeletal formula, the above formula can be represented as the formula shown in the options
Hence, the correct answer is Option $ (C) $ .
Note :
In the skeletal structure of a hydrocarbon, if at the end of the chain no group is mentioned, we have to always consider a methyl group at the end. In the skeletal structure, the lines show the bonds and the points of intersection of these straight lines or the nodes where the line changes direction shows the presence of carbon, and the hydrogen are balanced as per the remaining valency.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




