
The structure of di-chloromethane is
a.Tetrahedral
b.Trigonal
c.Linear
d.Hexagonal
Answer
527.4k+ views
Hint: We know that dichloromethane is an organochlorine compound with the formula $C{H_2}C{l_2}$. It is a colourless, volatile liquid with chloroform- like sweet odour is widely used as a solvent. We know that it is non-combustible but if exposed to high temperatures it may produce toxic chloride fumes.
Complete step by step answer:
We should know that the other name of di-chloromethane is Methylene chloride or Methylene dichloride.
The structure of dichloromethane is tetrahedral as it is $s{p^3}$ hybridized.
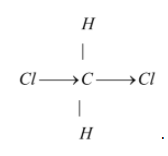
So it has a tetrahedral structure.
Hence the correct option is (a) tetrahedral.
Note:
We should know that even though $C{H_2}C{l_{12}}$ is not miscible in water, it can be dissolved in a wide range of organic solvents. This property of dichloromethane is combined with its volatility, making DCM a highly effective solvent in most industrial processes. It is also commonly used as a paint remover.
Complete step by step answer:
We should know that the other name of di-chloromethane is Methylene chloride or Methylene dichloride.
The structure of dichloromethane is tetrahedral as it is $s{p^3}$ hybridized.

So it has a tetrahedral structure.
Hence the correct option is (a) tetrahedral.
Note:
We should know that even though $C{H_2}C{l_{12}}$ is not miscible in water, it can be dissolved in a wide range of organic solvents. This property of dichloromethane is combined with its volatility, making DCM a highly effective solvent in most industrial processes. It is also commonly used as a paint remover.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




