
The structure of isobutyl group
A.
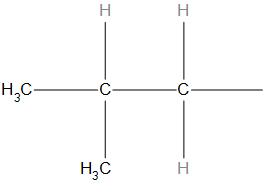
B.
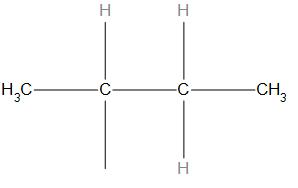
C.

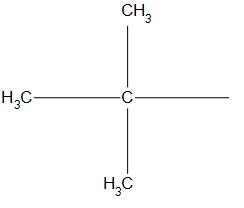
D.




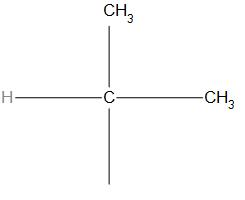
Answer
596.1k+ views
Hint: By removing a hydrogen from a primary carbon atom of isobutene gives a primary alkyl group which is called an isobutyl group. The Isobutyl group is having two methyl groups and one bond with hydrogen.
Step by step solution:- We can see the structure of the Isobutyl group, in which one carbon is having two methyl groups and one hydrogen bond must also be present. The iso prefix is described by the structure-
- In (b) structure, we can see that the substituent is attached to the secondary carbon (that is the carbon is attached to two adjacent carbons), so it is the structure of secondary butyl.
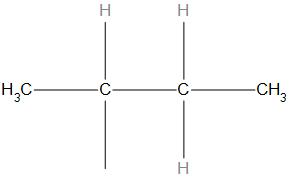
- In (c) structure, the substituent is attached to the last carbon in a chain hence it is a n butyl structure.

- In (d) structure, we can see that the substituent is attached to the tertiary carbon (that is the carbon to which three methyl group is present). Hence, it is a tertiary butyl structure.

-In (A) structure, the iso group is present hence, we can say that it is a structure of isobutyl.
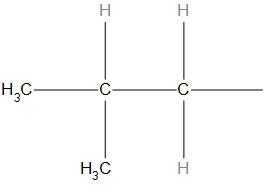
Hence, we can say that the option(A) is correct.
Additional information:We must be careful while using the various isobutyl compounds as it can cause harm-
- If this chemical contacts with the eyes, we must immediately wash our eyes with large amounts of water, by continuously lifting the lower and upper lids. We must get the medical attention immediately. We should not wear the contact lenses when working with this chemical.
- Skin: If this chemical contacts the skin, wash the contaminated skin with water continuously. If this chemical is dropped on the clothing, immediately remove the clothing If irritation persists even after washing, get medical attention.
- Breathing: If a person breathes large amounts of this chemical, then they must be moved to an open area with fresh air. Keep the affected person warm and at rest. Get medical attention as soon as possible.
Note:
- We must not get confused between the groups like isobutyl and other butyl groups like sec butyl, tertiary butyl groups and should be able to differentiate between them accordingly.
-sec butyl group means when the substituent is attached to the secondary carbon (that is the carbon is attached to two adjacent carbons).
- tertiary butyl groups means when the substituent is attached to the tertiary carbon (that is the carbon to which three methyl groups are present).
- Isobutyl group has two methyl groups and one bond with hydrogen.
Step by step solution:- We can see the structure of the Isobutyl group, in which one carbon is having two methyl groups and one hydrogen bond must also be present. The iso prefix is described by the structure-

- In (b) structure, we can see that the substituent is attached to the secondary carbon (that is the carbon is attached to two adjacent carbons), so it is the structure of secondary butyl.

- In (c) structure, the substituent is attached to the last carbon in a chain hence it is a n butyl structure.

- In (d) structure, we can see that the substituent is attached to the tertiary carbon (that is the carbon to which three methyl group is present). Hence, it is a tertiary butyl structure.

-In (A) structure, the iso group is present hence, we can say that it is a structure of isobutyl.

Hence, we can say that the option(A) is correct.
Additional information:We must be careful while using the various isobutyl compounds as it can cause harm-
- If this chemical contacts with the eyes, we must immediately wash our eyes with large amounts of water, by continuously lifting the lower and upper lids. We must get the medical attention immediately. We should not wear the contact lenses when working with this chemical.
- Skin: If this chemical contacts the skin, wash the contaminated skin with water continuously. If this chemical is dropped on the clothing, immediately remove the clothing If irritation persists even after washing, get medical attention.
- Breathing: If a person breathes large amounts of this chemical, then they must be moved to an open area with fresh air. Keep the affected person warm and at rest. Get medical attention as soon as possible.
Note:
- We must not get confused between the groups like isobutyl and other butyl groups like sec butyl, tertiary butyl groups and should be able to differentiate between them accordingly.
-sec butyl group means when the substituent is attached to the secondary carbon (that is the carbon is attached to two adjacent carbons).
- tertiary butyl groups means when the substituent is attached to the tertiary carbon (that is the carbon to which three methyl groups are present).
- Isobutyl group has two methyl groups and one bond with hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




