
The structure of \[PC{l_5}\] is
a.) Trigonal bipyramidal
b.) Octahedral
c.) Pentagonal bipyramidal
d.) Square pyramidal
Answer
557.4k+ views
Hint: For finding out the hybridization of the given molecule we can use the hybridization formula, and by referring to the arrangement of $spdf$ orbitals we can conclude the structure of the given molecular formula.
Complete step by step answer:
First consider which is the central atom, in this question our central atom is Phosphorous. Now, since the central atom has been found we will consider the hybridization formula.
- The general formula of hybridization is is given as
$H = (V + X + C + A)/2$
Where,
$V = $ The number of valence electrons of the central atom.
$X = $ The number of monovalent atoms attached to the central atom.
$C = $Total positive charge on the molecule.
$A = $ Total negative charge on the molecule.
- Now, for \[PC{l_5}\] since we know that central atom is phosphorous and there are $5$ chlorine atom surrounding it, and the number of valence electron of phosphorus is $5$, then writing all the values;
Total number of valence electrons of Phosphorus; $V = 5$
The number of fluorine attached to iodine; $X = 5$
Total positive charge on the molecule; $C = + 0$
Total negative charge on the molecule; $A = - 0$
By putting all the above given values in the hybridization formula we get,
$H = (5 + 5 + 0 - 0)/2$
$H = 10/2$,
$H = 5$,
Now referring to the table given below:
By considering the above table we get to know that since our $H$ is $5$ the hybridization of $PC{l_5}$ is trigonal bipyramidal.
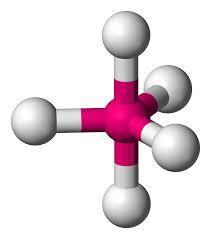
The correct answer is option “A” .
Note: In questions related to structures always remember to apply hybridization formula, and refer to the table given above, it is applicable to all compounds which have hybridization seven or less than it.
Complete step by step answer:
First consider which is the central atom, in this question our central atom is Phosphorous. Now, since the central atom has been found we will consider the hybridization formula.
- The general formula of hybridization is is given as
$H = (V + X + C + A)/2$
Where,
$V = $ The number of valence electrons of the central atom.
$X = $ The number of monovalent atoms attached to the central atom.
$C = $Total positive charge on the molecule.
$A = $ Total negative charge on the molecule.
- Now, for \[PC{l_5}\] since we know that central atom is phosphorous and there are $5$ chlorine atom surrounding it, and the number of valence electron of phosphorus is $5$, then writing all the values;
Total number of valence electrons of Phosphorus; $V = 5$
The number of fluorine attached to iodine; $X = 5$
Total positive charge on the molecule; $C = + 0$
Total negative charge on the molecule; $A = - 0$
By putting all the above given values in the hybridization formula we get,
$H = (5 + 5 + 0 - 0)/2$
$H = 10/2$,
$H = 5$,
Now referring to the table given below:
| VALUES OF H | HYBRIDIZATION | STRUCTURE |
| 2 | $sp$ | Linear |
| 3 | $s{p^2}$ | Trigonal planar |
| 4 | $s{p^3}$ | Tetrahedral |
| 5 | $s{p^3}d$ | Trigonal bipyramidal |
| 6 | $s{p^3}{d^2}$ | Octahedral |
| 7 | $s{p^3}{d^3}$ | Pentagonal bipyramidal |
By considering the above table we get to know that since our $H$ is $5$ the hybridization of $PC{l_5}$ is trigonal bipyramidal.

The correct answer is option “A” .
Note: In questions related to structures always remember to apply hybridization formula, and refer to the table given above, it is applicable to all compounds which have hybridization seven or less than it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




