
The structure of potassium ferrocyanide is:
A.Tetrahedral
B.Octahedral
C.Square planar
D.Linear
Answer
601.2k+ views
Hint:
Solve this question by breaking up the name of the compound. The given compound is a salt made up of potassium and ferrocyanide ions. Potassium is the cation, whereas cyanide is an anion.
Complete step by step answer:
Potassium ferrocyanide is an inorganic compound with the molecular formula – \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]. It occurs as a lemon-yellow monoclinic crystal and is therefore also known as yellow potash prussiate.
It is a coordination compound. From the name, we can decipher that the compound is made of a cation (potassium) and cation (ferrocyanide). Now, let us break the term ferrocyanide –
‘ferro’ stands for ferrous, i.e., the element iron with an oxidation state of +2.
‘cyanide’ stands for a compound made up of C and N which is triple bonded to each other. The structure of cyanide is as given below –
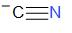
Cyanide has an overall charge = -1.
Therefore, the overall charge of ferrocyanide = (+2) + 6(-1) = -4.
From here, we can see, 4 potassium ions are needed to balance these.
The structure of potassium ferrocyanide can be drawn as –
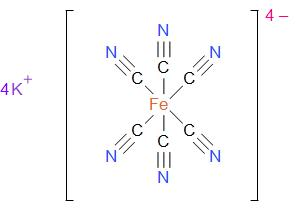
The geometry of the compound is dependent on ferrocyanide. A coordination compound with six ligands is always octahedral. It is known as the octahedral because it has eight faces.
Therefore, the answer is – option (b) – The structure of potassium ferrocyanide is octahedral.
Additional Information:
The IUPAC name of this compound is potassium hexacyanoferrate (II).
Note:
Do not confuse potassium ferrocyanide with potassium ferricyanide. In potassium ferricyanide, the iron atom has an oxidation state of +3. Iron is a d-block element with atomic number 26. It has two oxidation states - +2 (ferrous) and +3 (ferric).
Solve this question by breaking up the name of the compound. The given compound is a salt made up of potassium and ferrocyanide ions. Potassium is the cation, whereas cyanide is an anion.
Complete step by step answer:
Potassium ferrocyanide is an inorganic compound with the molecular formula – \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]. It occurs as a lemon-yellow monoclinic crystal and is therefore also known as yellow potash prussiate.
It is a coordination compound. From the name, we can decipher that the compound is made of a cation (potassium) and cation (ferrocyanide). Now, let us break the term ferrocyanide –
‘ferro’ stands for ferrous, i.e., the element iron with an oxidation state of +2.
‘cyanide’ stands for a compound made up of C and N which is triple bonded to each other. The structure of cyanide is as given below –
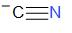
Cyanide has an overall charge = -1.
Therefore, the overall charge of ferrocyanide = (+2) + 6(-1) = -4.
From here, we can see, 4 potassium ions are needed to balance these.
The structure of potassium ferrocyanide can be drawn as –

The geometry of the compound is dependent on ferrocyanide. A coordination compound with six ligands is always octahedral. It is known as the octahedral because it has eight faces.
Therefore, the answer is – option (b) – The structure of potassium ferrocyanide is octahedral.
Additional Information:
The IUPAC name of this compound is potassium hexacyanoferrate (II).
Note:
Do not confuse potassium ferrocyanide with potassium ferricyanide. In potassium ferricyanide, the iron atom has an oxidation state of +3. Iron is a d-block element with atomic number 26. It has two oxidation states - +2 (ferrous) and +3 (ferric).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




