
The structure of \[S{F_4}\] is:
A. square planar
B. tetrahedral
C. see-saw structure
D. octahedral
Answer
561.6k+ views
Hint: \[S{F_4}\] is a chemical compound composed of sulphur and fluorine. It is an inorganic compound. The structure can be determined with the help of VSEPR theory.
Complete step by step answer:
VSEPR theory is the abbreviation of Valence Shell Electron Pair repulsion theory. This theory is used to determine the shape of the molecules by considering the number of electrons around the central atom.
As the name suggests, only the valence shell electrons are considered for elucidation of the structure. The theory says that the valence electrons whether bonded or non-bonded are arranged in such a way so as to minimize the electron pair repulsion. The repulsion varies in the order lone pair-lone pair > lone pair-bond pair > bond pair-bond pair electrons.
Out of sulphur and fluorine the less electronegative atom is sulphur, so it is the central atom of the given molecule. Sulphur is an atom in the periodic table with atomic number\[16\]. The electronic configuration of sulphur is
$S:1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$
The valence shell of sulphur is \[3\]which contain a total of \[6\]electrons, two in \[3s\] and four in\[3p\]. A total of four fluorine atoms are bonded to the central atom by sharing one electron. So the four electrons of the central atom bonded to four fluorine atoms by sharing one electron each between the bonded atoms leaving behind two electrons non-bonded.
The VSEP number = \[\dfrac{1}{2}\] (number of electrons around the central atom + number of electron from four fluorine atoms)
\[ = \dfrac{1}{2}\left( {6 + 4} \right) = 5\] .
The VSEP number equal to five belongs to the shape trigonal bipyramidal. Out of the five corners of the geometry one is occupied by the lone pair of electrons of the central atom sulphur. The other four corners are occupied by the four fluorine atoms. Thus the structure of \[S{F_4}\] is see-saw structure, i.e. option C is the correct answer.
The structure of \[S{F_4}\] is shown as:
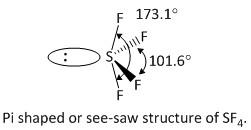
So, the correct answer is Option C.
Note: The VSEPR theory fails to explain the structure of transition metal complexes as it did not consider the size of the substituents attached to the central atom. Knowing the geometry of the molecule helps in understanding the reactions of the molecule.
Complete step by step answer:
VSEPR theory is the abbreviation of Valence Shell Electron Pair repulsion theory. This theory is used to determine the shape of the molecules by considering the number of electrons around the central atom.
As the name suggests, only the valence shell electrons are considered for elucidation of the structure. The theory says that the valence electrons whether bonded or non-bonded are arranged in such a way so as to minimize the electron pair repulsion. The repulsion varies in the order lone pair-lone pair > lone pair-bond pair > bond pair-bond pair electrons.
Out of sulphur and fluorine the less electronegative atom is sulphur, so it is the central atom of the given molecule. Sulphur is an atom in the periodic table with atomic number\[16\]. The electronic configuration of sulphur is
$S:1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$
The valence shell of sulphur is \[3\]which contain a total of \[6\]electrons, two in \[3s\] and four in\[3p\]. A total of four fluorine atoms are bonded to the central atom by sharing one electron. So the four electrons of the central atom bonded to four fluorine atoms by sharing one electron each between the bonded atoms leaving behind two electrons non-bonded.
The VSEP number = \[\dfrac{1}{2}\] (number of electrons around the central atom + number of electron from four fluorine atoms)
\[ = \dfrac{1}{2}\left( {6 + 4} \right) = 5\] .
The VSEP number equal to five belongs to the shape trigonal bipyramidal. Out of the five corners of the geometry one is occupied by the lone pair of electrons of the central atom sulphur. The other four corners are occupied by the four fluorine atoms. Thus the structure of \[S{F_4}\] is see-saw structure, i.e. option C is the correct answer.
The structure of \[S{F_4}\] is shown as:

So, the correct answer is Option C.
Note: The VSEPR theory fails to explain the structure of transition metal complexes as it did not consider the size of the substituents attached to the central atom. Knowing the geometry of the molecule helps in understanding the reactions of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




