
The sulphur molecule ${{\text{S}}_{\text{8}}}$ can be represented as:
A.Cubical structure
B.Spherical structure
C.Tetrahedral structure
D.W-shaped ring structure
Answer
585.6k+ views
Hint: ${{\text{S}}_{\text{8}}}$ molecule has eight sulphur molecules bonded with each other with a single covalent bond with each sulphur having a covalency of 2 and oxidation state being zero and forms a closed crown-shaped structure or a circular structure.
Complete step by step answer:
Now let us look into this sulphur molecule in a more elaborate way:
-It is important for all of you to know that, ${{\text{S}}_{\text{8}}}$ Octasulfur is an inorganic chemical. It is yellow in colour, is odourless and tasteless. It is the most common allotrope of sulphur.
-The ${{\text{S}}_{\text{8}}}$molecule of sulphur adopts a crown-shaped conformation which is sometimes also known as W-shaped ring structure with each sulphur bonded to one-another with a single covalent bond as shown in the following diagram:
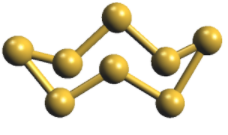
Hence, the correct answer is Option (D)
Additional information: As you all are aware that sulphur is a non-metal, having the atomic number 16 and atomic weight of 32 u. At present Sulphur has 30 well characterized isotopes in all. And no other element forms solid allotropes more than sulphur. Out of all these allotropes of sulphur rhombic sulphur is the most stable form of sulphur at room temperature.
Note:
We have mentioned the term allotrope in our discussion, so it is worth knowing about it. There is a property called allotropy which is the property of chemical elements to exist in more than one forms, which differ from each other in terms of physical states and these different forms of the elements are known as its allotropes.
Complete step by step answer:
Now let us look into this sulphur molecule in a more elaborate way:
-It is important for all of you to know that, ${{\text{S}}_{\text{8}}}$ Octasulfur is an inorganic chemical. It is yellow in colour, is odourless and tasteless. It is the most common allotrope of sulphur.
-The ${{\text{S}}_{\text{8}}}$molecule of sulphur adopts a crown-shaped conformation which is sometimes also known as W-shaped ring structure with each sulphur bonded to one-another with a single covalent bond as shown in the following diagram:

Hence, the correct answer is Option (D)
Additional information: As you all are aware that sulphur is a non-metal, having the atomic number 16 and atomic weight of 32 u. At present Sulphur has 30 well characterized isotopes in all. And no other element forms solid allotropes more than sulphur. Out of all these allotropes of sulphur rhombic sulphur is the most stable form of sulphur at room temperature.
Note:
We have mentioned the term allotrope in our discussion, so it is worth knowing about it. There is a property called allotropy which is the property of chemical elements to exist in more than one forms, which differ from each other in terms of physical states and these different forms of the elements are known as its allotropes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




