
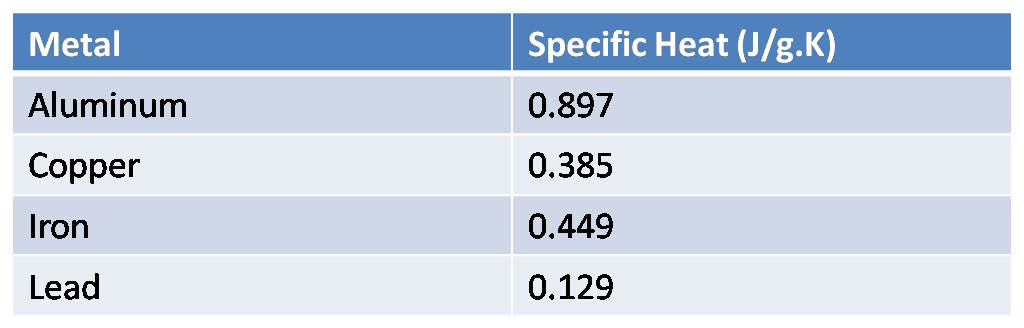
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from $20{}^\circ C$ to $30{}^\circ C$ when it absorbs 67.5 J of heat. Which of the metals in the table below could be the unknown metal?

Answer
502.8k+ views
Hint: Specific heat tells us about heat needed for the rise in temperature by one degree Celsius by one gram of any element. Specific heat and heat are two different entities related to each other.
Formula used:
Formula for heat energy, Q=$mc\Delta T$
Complete answer:
We are given the specific heats of various metals in the table. Given the data that,
Heat absorbed = 67.5 J
Mass of the sample = 15 g
Temperatures = $20{}^\circ C$to$30{}^\circ C$ so, $\Delta T$= 30 – 20 = $10{}^\circ C$
We have to find from this given data, the metal from the table that possesses the specific heat as obtained by this given information.
As formula for heat absorbed is Q=$mc\Delta T$
Keeping the given data in this formula, we get:
67.5 = 10g$c\times 10{}^\circ C$
c = $\dfrac{67.5\,J}{15\,g\times 10\,{}^\circ C}$
c = 0.45 J/ g.K
This value of specific heat is around the specific heat of iron from the table.
Hence, iron metal from the table could be the unknown metal.
Note:
The temperature difference, $\Delta T$, taken out in Celsius will be the same as taken out for Kelvin. The specific heat in the answer does not match any quantity in the table, so we use that quantity of specific heat which is most close to the calculated value, therefore, iron is the answer.
Formula used:
Formula for heat energy, Q=$mc\Delta T$
Complete answer:
We are given the specific heats of various metals in the table. Given the data that,
Heat absorbed = 67.5 J
Mass of the sample = 15 g
Temperatures = $20{}^\circ C$to$30{}^\circ C$ so, $\Delta T$= 30 – 20 = $10{}^\circ C$
We have to find from this given data, the metal from the table that possesses the specific heat as obtained by this given information.
As formula for heat absorbed is Q=$mc\Delta T$
Keeping the given data in this formula, we get:
67.5 = 10g$c\times 10{}^\circ C$
c = $\dfrac{67.5\,J}{15\,g\times 10\,{}^\circ C}$
c = 0.45 J/ g.K
This value of specific heat is around the specific heat of iron from the table.
Hence, iron metal from the table could be the unknown metal.
Note:
The temperature difference, $\Delta T$, taken out in Celsius will be the same as taken out for Kelvin. The specific heat in the answer does not match any quantity in the table, so we use that quantity of specific heat which is most close to the calculated value, therefore, iron is the answer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




