
The target used in Coolidge tube used for the production of X-rays is made up of________
A. manganese
B. molybdenum
C. uranium
D. radium
Answer
592.2k+ views
Hint: X-rays are a type of radiation called electromagnetic waves. The modern technology consists of Coolidge tubes for the production of X-rays. For the production of X-rays, the target should have a high atomic number along with good heat storage capacity and low rate of evaporation.
Complete step by step answer:
An X-radiation is a penetrating form of high energy electromagnetic radiation. They usually have a wavelength ranging from \[10\text{ picometres}\] to \[\text{10 nanometres}\] and corresponding frequencies in the range of $30\text{ petahertz}$ to $30\text{ exahertz}$, that is, $3\times {{10}^{15}}Hz$ to $3\times {{10}^{18}}Hz$. The energy of X-rays lies in the range $124eV$ to $124KeV$.
X-rays are produced when the fast moving electrons strike a metal target of suitable material.
The basic requirements for the production of X-rays are:
A source of electrons
Effective means of accelerating the electrons and
A target of suitable material and high atomic weight.
The modern type of X-ray tube designed by Coolidge consists of a highly evacuated hard glass bulb containing a cathode and an anode target. The cathode target is heated by passing electric current through it from a low tension battery. Anode is the component in which X-rays are produced. The function of anode is to convert the electricity into X-rays and dissipate the heat in the process. For this purpose, material such as molybdenum is used with a high atomic number which has good heat storage capacity and low rate of evaporation.
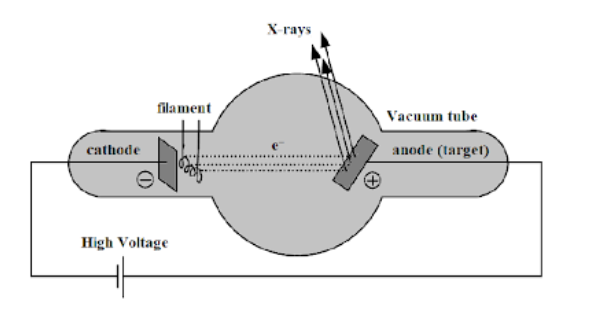
The electrons are emitted by the process called as thermionic emission from the cathode. The filament of the tube is surrounded by a molybdenum cylinder kept at a negative potential to the filament. Thus, the electrons emitted from the filament are collimated into a fine pencil of electron beam. This fine electron beam is called X-rays.
Molybdenum is used as a target in Coolidge tubes for the production of X-rays.
Hence, the correct option is B.
Note:
The Coolidge tube has anode and cathode targets. The anode is the component in which X-rays are produced. The intensity of X-rays depends upon the total number of electrons striking the target, that is, the rate of emission of electrons from the cathode filament. This can be controlled by varying the amount of filament current.
Complete step by step answer:
An X-radiation is a penetrating form of high energy electromagnetic radiation. They usually have a wavelength ranging from \[10\text{ picometres}\] to \[\text{10 nanometres}\] and corresponding frequencies in the range of $30\text{ petahertz}$ to $30\text{ exahertz}$, that is, $3\times {{10}^{15}}Hz$ to $3\times {{10}^{18}}Hz$. The energy of X-rays lies in the range $124eV$ to $124KeV$.
X-rays are produced when the fast moving electrons strike a metal target of suitable material.
The basic requirements for the production of X-rays are:
A source of electrons
Effective means of accelerating the electrons and
A target of suitable material and high atomic weight.
The modern type of X-ray tube designed by Coolidge consists of a highly evacuated hard glass bulb containing a cathode and an anode target. The cathode target is heated by passing electric current through it from a low tension battery. Anode is the component in which X-rays are produced. The function of anode is to convert the electricity into X-rays and dissipate the heat in the process. For this purpose, material such as molybdenum is used with a high atomic number which has good heat storage capacity and low rate of evaporation.

The electrons are emitted by the process called as thermionic emission from the cathode. The filament of the tube is surrounded by a molybdenum cylinder kept at a negative potential to the filament. Thus, the electrons emitted from the filament are collimated into a fine pencil of electron beam. This fine electron beam is called X-rays.
Molybdenum is used as a target in Coolidge tubes for the production of X-rays.
Hence, the correct option is B.
Note:
The Coolidge tube has anode and cathode targets. The anode is the component in which X-rays are produced. The intensity of X-rays depends upon the total number of electrons striking the target, that is, the rate of emission of electrons from the cathode filament. This can be controlled by varying the amount of filament current.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




