
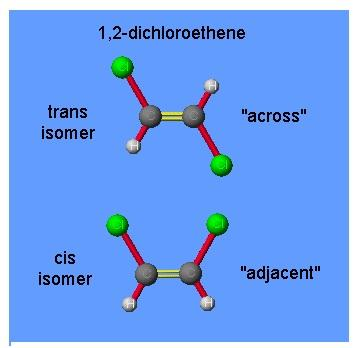
The two images of 1, 2-dichloroethene are attached to this question. Can we call them isomers?
A.Yes
B.No
C.Maybe
D.Can’t say

Answer
582.3k+ views
Hint:These two molecules in the picture are not the same. The carbon-carbon double bond won't rotate and so we have to take the models to pieces in order to convert one structure into the other one. That is a simple test for isomers. If we take a model to pieces to convert it into another one, then we have got isomers.
Complete step by step answer:
The two molecules are the two isomers of 1,2-dichloroethene. The first molecule, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. The word trans comes from the Latin word which means ‘across’.
The second molecule, the two chlorine atoms are locked on the same side of the double bond. This is known as the cis isomer. The word cis comes from the Latin word which means ‘on this side’.The first molecule is the trans isomer and the second molecule is the cis isomer.
So, yes, these two can be called as isomers.
Therefore, the correct answer is option (A).
Note: Isomers are the molecules whose molecular formula are same, but the arrangement of the atoms in space is different. That will exclude any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. 1,2-Dichloroethene is commonly called 1,2-dichloroethylene or 1,2-DCE. It is an organochlorine with the molecular formula ${C_2}{H_2}C{I_2}$ . 1,2-Dichloroethene is a highly flammable, and colourless liquid with a sharp, harsh smell.
Complete step by step answer:
The two molecules are the two isomers of 1,2-dichloroethene. The first molecule, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. The word trans comes from the Latin word which means ‘across’.
The second molecule, the two chlorine atoms are locked on the same side of the double bond. This is known as the cis isomer. The word cis comes from the Latin word which means ‘on this side’.The first molecule is the trans isomer and the second molecule is the cis isomer.
So, yes, these two can be called as isomers.
Therefore, the correct answer is option (A).
Note: Isomers are the molecules whose molecular formula are same, but the arrangement of the atoms in space is different. That will exclude any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. 1,2-Dichloroethene is commonly called 1,2-dichloroethylene or 1,2-DCE. It is an organochlorine with the molecular formula ${C_2}{H_2}C{I_2}$ . 1,2-Dichloroethene is a highly flammable, and colourless liquid with a sharp, harsh smell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




