
The valency of iron in $F{e_2}{O_3}$is
A. 1
B. 2
C. 3
D. 6
Answer
576.9k+ views
Hint: Valency is defined as the combining power of the element with the other element when they combine to form a chemical compound. The octet rule is used to determine the valency of the atom in the chemical compound.
Complete step by step answer:
Valency is defined as the number of hydrogen atoms which combines or replaces the other atom of the atom to form a chemical compound.
The given chemical compound is $F{e_2}{O_3}$. The name of the compound is ferric oxide.
The atomic number of iron is 26. Its electronic configuration is $[Ar]3{d^6}4{s^2}$
S.V.S.D.F rule is used to determine the chemical formula of the compound, In this rule the valency of the compound is written below the element and then it is swapped to form the chemical formula.
Ferric oxide is represented as shown below.
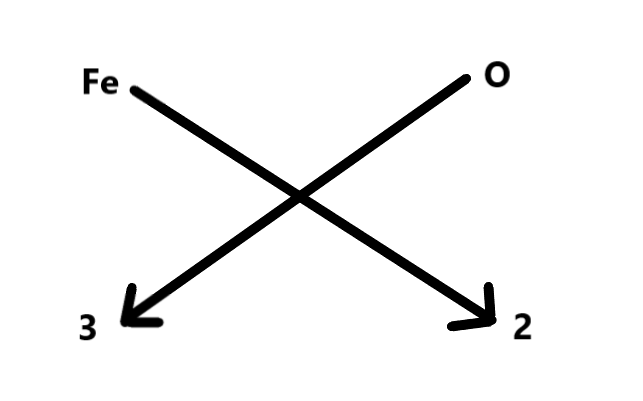
From this, we can find out that the valency of Fe is 3.
As the outermost orbital of iron contains two electrons, therefore it has positive valency.
Therefore, the correct option is C .
Note: The valency of any atom in the chemical compound is calculated by using octet rule. According to the rule, a compound is considered as a stable compound when its outermost orbital consists of eight electrons.When the number of electrons present in the outermost orbital varies from one to four, the compound carries positive valency. The compound carries electrons from four to seven in the outermost orbital, its valency is determined by subtracting the value with eight.
Complete step by step answer:
Valency is defined as the number of hydrogen atoms which combines or replaces the other atom of the atom to form a chemical compound.
The given chemical compound is $F{e_2}{O_3}$. The name of the compound is ferric oxide.
The atomic number of iron is 26. Its electronic configuration is $[Ar]3{d^6}4{s^2}$
S.V.S.D.F rule is used to determine the chemical formula of the compound, In this rule the valency of the compound is written below the element and then it is swapped to form the chemical formula.
Ferric oxide is represented as shown below.

From this, we can find out that the valency of Fe is 3.
As the outermost orbital of iron contains two electrons, therefore it has positive valency.
Therefore, the correct option is C .
Note: The valency of any atom in the chemical compound is calculated by using octet rule. According to the rule, a compound is considered as a stable compound when its outermost orbital consists of eight electrons.When the number of electrons present in the outermost orbital varies from one to four, the compound carries positive valency. The compound carries electrons from four to seven in the outermost orbital, its valency is determined by subtracting the value with eight.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




