
The valency of metal whose sulfate is $MS{O_4}$ is 2.
A. True
B. False
Answer
607.8k+ views
Hint: These are the basic questions to check your preparation in basic concepts, so remember the basic structure of sulfate, valency, and also the bond formation in sulfate to find if the statement mentioned in the question is true or false.
Complete answer:
We know that valency is defined as the number of electrons present in valence shells that are being lost, gained, or shared with other atoms to form molecules or to gain stability.
And the valency of sulfate ion i.e. $S{O_4}$ is −2 considering the image below
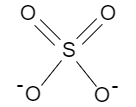
Thus, a metal which combines with it should have +2 valence electrons to accept 2 electrons from sulfate ions.
Hence, the statement mentioned in the question is true.
Note: Sulfates are sulfate anion-containing chemical compounds ($SO_4^{2 - }$) these are sulfuric acid salts or esters, \[{H_2}S{O_4}\]which are formed by a metal cation or organic group replacing one or both of the hydrogen atoms. Most metal sulfates are readily soluble in water, but only partially soluble is calcium sulfate, while insoluble in barium, lead, and strontium sulfate. Sulfate is a naturally occurring compound. This exists in varying quantities, naturally in water. When there is a high sulfate content in water, the water may have a bitter taste. There are also sulfates present in minerals, soil, rocks, plants, and food. Sulfates are chemical compounds that are used as cleaning agents. They are used in cleaners, detergents, and even shampoos in households. The shampoo contains two main types of sulfates: sodium lauryl sulfate, and sodium Laureth sulfate. Such sulfates are meant to produce a lathering effect to remove the oil and dirt from your hair.
Complete answer:
We know that valency is defined as the number of electrons present in valence shells that are being lost, gained, or shared with other atoms to form molecules or to gain stability.
And the valency of sulfate ion i.e. $S{O_4}$ is −2 considering the image below

Thus, a metal which combines with it should have +2 valence electrons to accept 2 electrons from sulfate ions.
Hence, the statement mentioned in the question is true.
Note: Sulfates are sulfate anion-containing chemical compounds ($SO_4^{2 - }$) these are sulfuric acid salts or esters, \[{H_2}S{O_4}\]which are formed by a metal cation or organic group replacing one or both of the hydrogen atoms. Most metal sulfates are readily soluble in water, but only partially soluble is calcium sulfate, while insoluble in barium, lead, and strontium sulfate. Sulfate is a naturally occurring compound. This exists in varying quantities, naturally in water. When there is a high sulfate content in water, the water may have a bitter taste. There are also sulfates present in minerals, soil, rocks, plants, and food. Sulfates are chemical compounds that are used as cleaning agents. They are used in cleaners, detergents, and even shampoos in households. The shampoo contains two main types of sulfates: sodium lauryl sulfate, and sodium Laureth sulfate. Such sulfates are meant to produce a lathering effect to remove the oil and dirt from your hair.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




