
The valency of the carbonate radical is :
A. 1
B. 2
C. 3
D. 4
Answer
585.3k+ views
Hint: The capacity of an atom to combine with other atoms to form a molecule is known as its valency. The number of bonds that an atom forms with other atoms is going to depend on the valency of the particular atom.
Complete step by step answer:
- The given molecule in the question is carbonate radical.
- To know the valency of any molecule we should know the structure of it.
- The structure of carbonate radical is as follows.
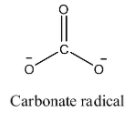
- We can see that the carbonate radical contains three oxygen atoms in its structure.
- One oxygen atom is bonded to carbon through a double bond and the remaining two oxygen atoms are bonded to carbon through a single bond and contain a negative charge on them.
- Oxygen has one negative charge means it combines with one another atom.
- In the given compound there are two oxygen atoms having two negative charges means they combine with two other atoms to get neutralized.
- Therefore the valency of the carbonate radical is two.
- So, the correct option is B.
Note: Carbonate radical combines easily with two hydrogen ions and forms carbonic acid.
We can represent the carbonate radical as $CO_{3}^{2-}$ .
The reaction of carbonate ion $CO_{3}^{2-}$ with two hydrogens ions as follows.
\[CO_{3}^{2-}+2{{H}^{+}}\to \underset{Carbonic\text{ }acid}{\mathop{{{H}_{2}}C{{O}_{3}}}}\,\]
The valency of bicarbonate ions is one.
We can represent the bicarbonate as $HCO_{3}^{-}$ . Since bicarbonate has one negative charge it combines with one other atom. So, the valency of the bicarbonate is one.
Complete step by step answer:
- The given molecule in the question is carbonate radical.
- To know the valency of any molecule we should know the structure of it.
- The structure of carbonate radical is as follows.

- We can see that the carbonate radical contains three oxygen atoms in its structure.
- One oxygen atom is bonded to carbon through a double bond and the remaining two oxygen atoms are bonded to carbon through a single bond and contain a negative charge on them.
- Oxygen has one negative charge means it combines with one another atom.
- In the given compound there are two oxygen atoms having two negative charges means they combine with two other atoms to get neutralized.
- Therefore the valency of the carbonate radical is two.
- So, the correct option is B.
Note: Carbonate radical combines easily with two hydrogen ions and forms carbonic acid.
We can represent the carbonate radical as $CO_{3}^{2-}$ .
The reaction of carbonate ion $CO_{3}^{2-}$ with two hydrogens ions as follows.
\[CO_{3}^{2-}+2{{H}^{+}}\to \underset{Carbonic\text{ }acid}{\mathop{{{H}_{2}}C{{O}_{3}}}}\,\]
The valency of bicarbonate ions is one.
We can represent the bicarbonate as $HCO_{3}^{-}$ . Since bicarbonate has one negative charge it combines with one other atom. So, the valency of the bicarbonate is one.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




