
The various types of hydrides and examples of each type are given below:
Choose the correct matching from the codes given below.
HYDRIDE TYPES COMPOUNDS Electron deficient LiH Saline \[C{H_4}\] Electron precise \[N{H_3}\] Interstitial \[{B_2}{H_6}\] lectron rich CrH
А. (A) - (ii), (B) - (iv), (C) – (v), (D) - (iii), (E) - ()
B. (A) - (iv), (B) - (i), (C) - (i), (D) (v), (E) - (iii)
C. (A) - (iv), (B) - (iii), (C) (v), (D) – (i), (E) – (i)
D. (A) - (v), (B) – (iii), (C) – (iv), (D) – (ii), (E) – (i)
| HYDRIDE TYPES | COMPOUNDS |
| Electron deficient | LiH |
| Saline | \[C{H_4}\] |
| Electron precise | \[N{H_3}\] |
| Interstitial | \[{B_2}{H_6}\] |
| lectron rich | CrH |
Answer
580.8k+ views
Hint: To solve this question, we must first understand the different types of hydrides and their definitions and properties. Then we must correlate the characteristic to the hydrides given to us via their Lewis Structures and properties.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
-Electron deficient compounds:
Electron deficient compounds are formed when the valence shell of the central atom does not have lone pairs that the central atom can donate, instead it has space for accepting lone pairs of electrons. So basically, these compounds are Lewis acids.
-Saline Hydrides:
Saline Hydrides are those hydrides that are formed in ionic compounds. These hydrides are also known as pseudo halides or ionic hydrides. These hydrides are usually formed between hydrogen and either alkali or alkali earth metals.
-Electron precise compounds:
Electron precise compounds have exactly 8 electrons in their valence shell, thus completing the octet.
-Interstitial hydrides:
Interstitial Hydrides are between hydrogen and transition metals. The hydrogen atoms are placed at positions of crystal defect in these compounds, hence their stoichiometric ratios for hydrogen are not fixed.
-Electron rich compounds:
Electron rich compounds are formed when the valence shell of the central atom has lone pairs that the central atom can donate. So basically, these compounds are Lewis bases.
-Now let us move towards the hydrides given to us:
LiH is formed when atoms of hydrogen and lithium combine together. They form bonds by transfer of electrons from lithium to hydrogen. Hence, it is an ionic compound. Hence, LiH can be considered to be a saline hydride.
Chromium is a transition metal. Hence the hydride formed by chromium would be an interstitial hydride. Hence, CrH is an interstitial hydride.
The Lewis structure of methane can be given as:
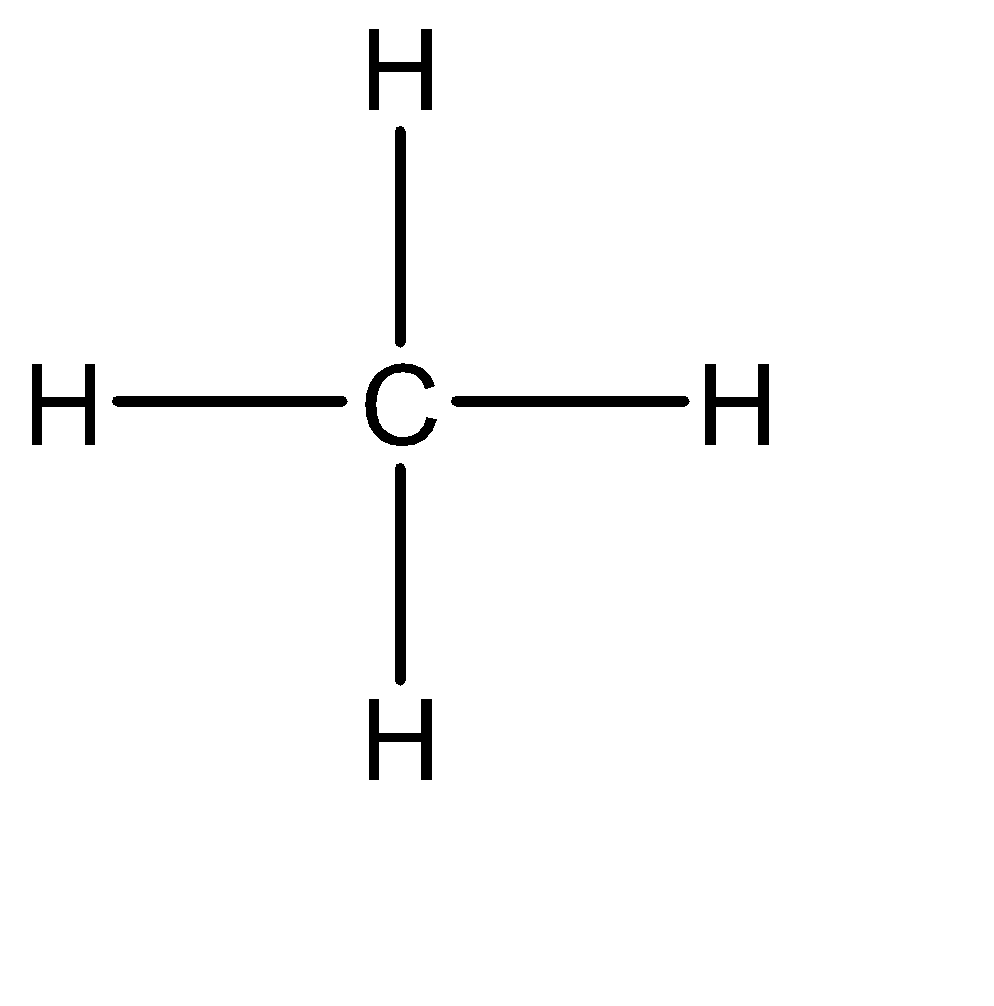
The electron configuration of carbon is \[1{s^2}2{s^2}2{p^2}\] , and there are 4 bonds formed. Hence, there are 8 electrons in the valence shell of carbon, and hence, it is an electron precise hydride.
The Lewis structure for \[N{H_3}\] is:
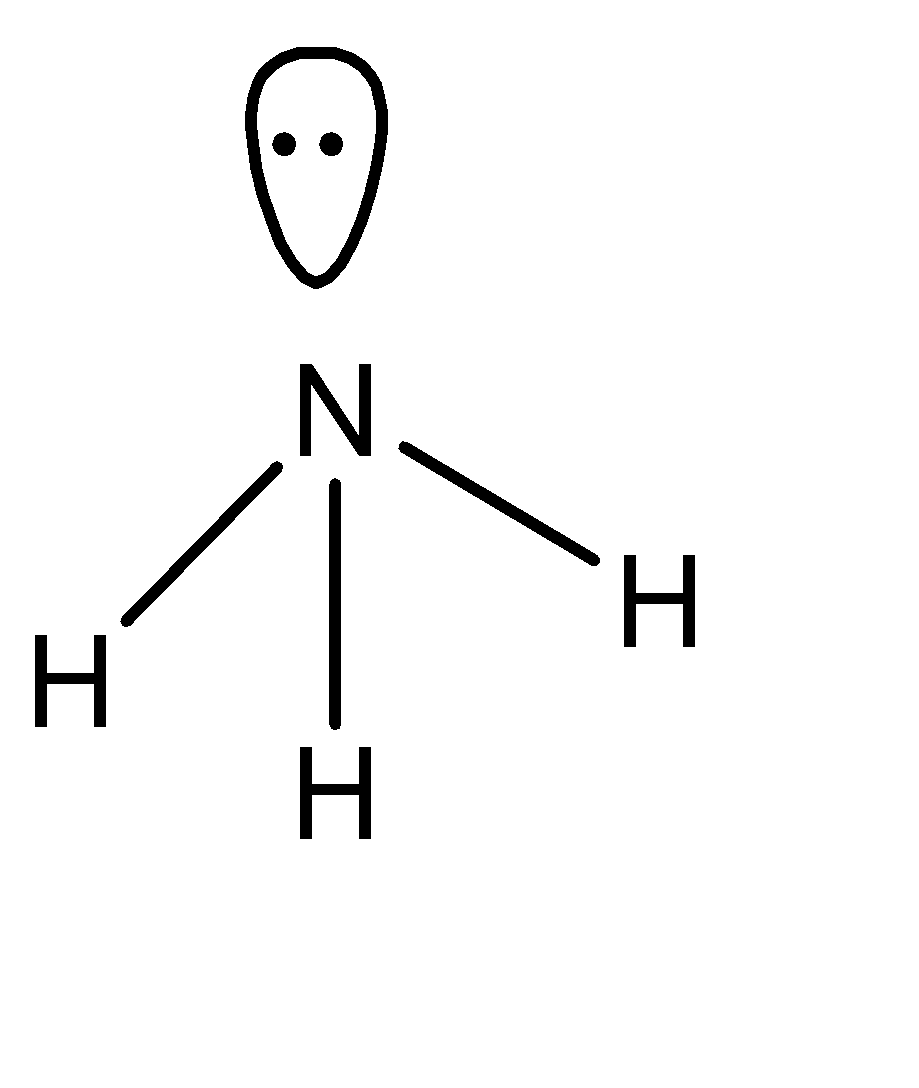
The electron configuration of nitrogen is \[1{s^2}2{s^2}2{p^3}\] , and there are 3 bonds and 1 lone pair of electrons. Hence there is a lone pair that can be donated. Because of this ammonia is electron rich hydride.
The Lewis structure for \[{B_2}{H_6}\] is:
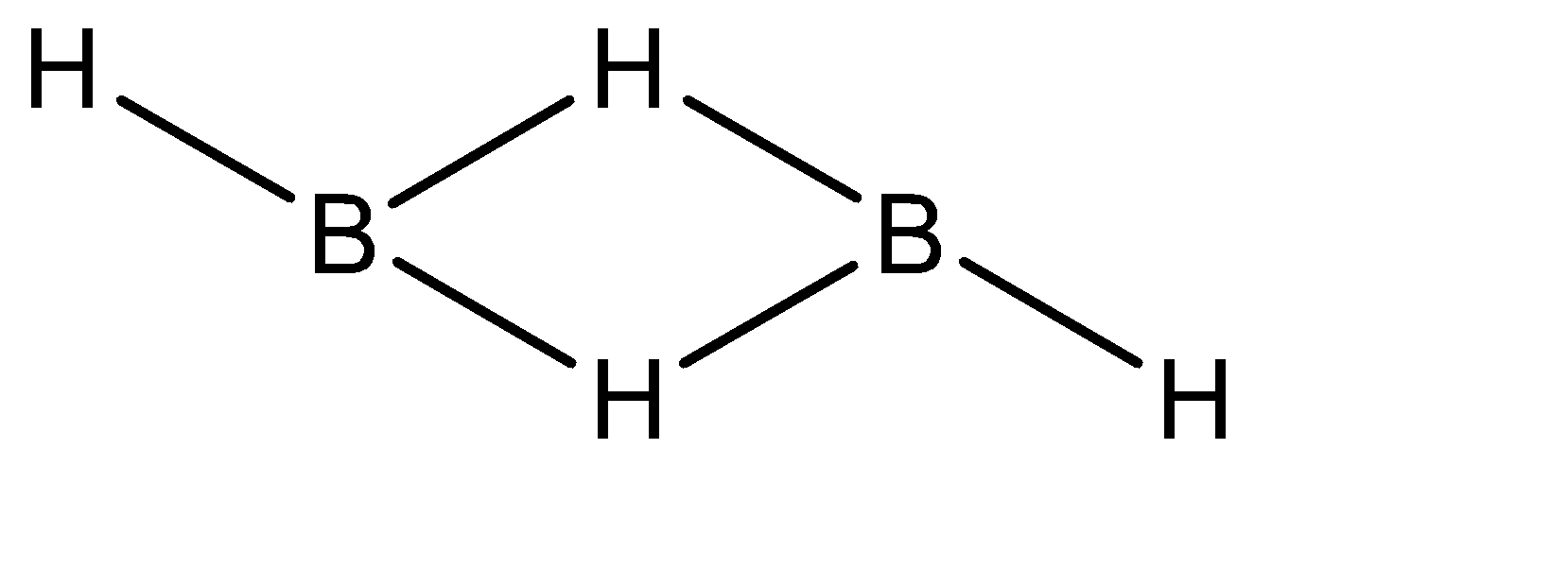
The electron configuration of boron is \[1{s^2}2{s^2}2{p^1}\] , and there are 3 bonds and 0 lone pairs of electrons. Hence there are 6 electrons in the valence shell of diborane. Because of this diborane is electron deficient hydride.
Hence, Option B is the correct option.
Note:
Interstitial hydrides which are also called metallic hydrides are those in which the oxidation number of hydrogens is zero. Palladium, Uranium etc atoms form these types of hydrides.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
-Electron deficient compounds:
Electron deficient compounds are formed when the valence shell of the central atom does not have lone pairs that the central atom can donate, instead it has space for accepting lone pairs of electrons. So basically, these compounds are Lewis acids.
-Saline Hydrides:
Saline Hydrides are those hydrides that are formed in ionic compounds. These hydrides are also known as pseudo halides or ionic hydrides. These hydrides are usually formed between hydrogen and either alkali or alkali earth metals.
-Electron precise compounds:
Electron precise compounds have exactly 8 electrons in their valence shell, thus completing the octet.
-Interstitial hydrides:
Interstitial Hydrides are between hydrogen and transition metals. The hydrogen atoms are placed at positions of crystal defect in these compounds, hence their stoichiometric ratios for hydrogen are not fixed.
-Electron rich compounds:
Electron rich compounds are formed when the valence shell of the central atom has lone pairs that the central atom can donate. So basically, these compounds are Lewis bases.
-Now let us move towards the hydrides given to us:
LiH is formed when atoms of hydrogen and lithium combine together. They form bonds by transfer of electrons from lithium to hydrogen. Hence, it is an ionic compound. Hence, LiH can be considered to be a saline hydride.
Chromium is a transition metal. Hence the hydride formed by chromium would be an interstitial hydride. Hence, CrH is an interstitial hydride.
The Lewis structure of methane can be given as:

The electron configuration of carbon is \[1{s^2}2{s^2}2{p^2}\] , and there are 4 bonds formed. Hence, there are 8 electrons in the valence shell of carbon, and hence, it is an electron precise hydride.
The Lewis structure for \[N{H_3}\] is:

The electron configuration of nitrogen is \[1{s^2}2{s^2}2{p^3}\] , and there are 3 bonds and 1 lone pair of electrons. Hence there is a lone pair that can be donated. Because of this ammonia is electron rich hydride.
The Lewis structure for \[{B_2}{H_6}\] is:

The electron configuration of boron is \[1{s^2}2{s^2}2{p^1}\] , and there are 3 bonds and 0 lone pairs of electrons. Hence there are 6 electrons in the valence shell of diborane. Because of this diborane is electron deficient hydride.
Hence, Option B is the correct option.
Note:
Interstitial hydrides which are also called metallic hydrides are those in which the oxidation number of hydrogens is zero. Palladium, Uranium etc atoms form these types of hydrides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




