
Why is there an absorption of energy in certain chemical reactions that release of energy in other reactions?
Answer
569.4k+ views
Hint: Basically, there are two types of reaction. The first is an endothermic reaction while the second is an exothermic reaction. The primary difference between these two reactions is that one absorbs the heat from the surrounding while the other releases the heat.
Complete answer:
- The endothermic reactions are the chemical reactions in which the reactants absorb heat energy from the surroundings to form products whereas in case of exothermic reaction, the heat is released. Basically, if a reactant molecule in a particular reaction has more energy than the product molecules then the energy is released and this type of reaction is known as exothermic reaction.
- However, if the product molecules have more energy than the reactants, then the reaction absorbs energy from the surroundings and this type of reaction is known as endothermic reaction.
- Now, the activation energy refers to the energy that must be provided to the reactants so that they can overcome the energy barrier and react. The energy level diagrams for both the reactions are as shown:
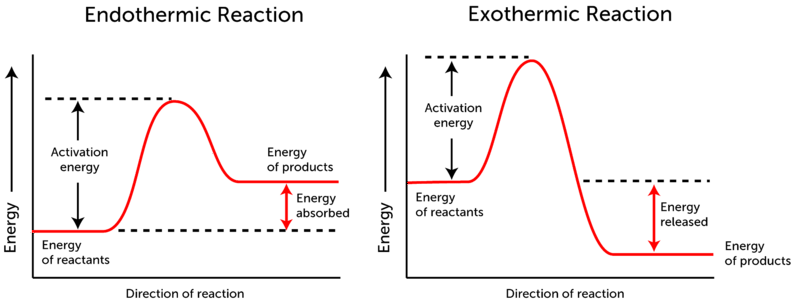
In case of exothermic reactions, the potential energy of the product is generally lower than that of the reactant but on the other hand, in case of endothermic reaction, the potential energy of the product is higher than that of reactants.
Note: Some of the endothermic reactions are melting of ice to form water, baking of bread, sublimation of solid $C{O_2}$ etc. Moreover, some examples of exothermic reactions are combustion reactions, rusting of iron, thermite reaction, dissolving laundry detergents in water etc.
Complete answer:
- The endothermic reactions are the chemical reactions in which the reactants absorb heat energy from the surroundings to form products whereas in case of exothermic reaction, the heat is released. Basically, if a reactant molecule in a particular reaction has more energy than the product molecules then the energy is released and this type of reaction is known as exothermic reaction.
- However, if the product molecules have more energy than the reactants, then the reaction absorbs energy from the surroundings and this type of reaction is known as endothermic reaction.
- Now, the activation energy refers to the energy that must be provided to the reactants so that they can overcome the energy barrier and react. The energy level diagrams for both the reactions are as shown:

In case of exothermic reactions, the potential energy of the product is generally lower than that of the reactant but on the other hand, in case of endothermic reaction, the potential energy of the product is higher than that of reactants.
Note: Some of the endothermic reactions are melting of ice to form water, baking of bread, sublimation of solid $C{O_2}$ etc. Moreover, some examples of exothermic reactions are combustion reactions, rusting of iron, thermite reaction, dissolving laundry detergents in water etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




