
Why is there greater repulsion between two lone pairs of electrons than between a lone pair and a bonding pair of electrons?
Answer
495k+ views
Hint: The idea of a lone pair is utilised in the valence shell electron pair repulsion theory (VSEPR theory), which explains molecular structures. They're also referred to in Lewis acid and base chemistry. Chemists, on the other hand, do not regard all non-bonding pairs of electrons to be lone pairs. With atoms in the nitrogen group, such as nitrogen in ammonia, a single lone pair may be discovered, two lone pairs can be found with atoms in the chalcogen group, such as oxygen in water, and halogens can carry three lone pairs, as in hydrogen chloride.
Complete answer:
Because the electrons in a lone pair are not involved in bonding and are thus free, the repulsion is higher. Because of one free electron, the repulsion between a lone pair and a bond pair is somewhat higher than that between a bond pair and a bond pair. It's because the orbitals that house lone pairs have a distinct form. The repulsion between two lone pairs of electrons is, in fact, higher than the repulsion between a lone pair and a bonded pair of electrons. The repulsion between a lone pair of electrons and a bonding pair of electrons is also larger than the repulsion between two bonding pairs of electrons.
The geometry of the orbitals that hold these lengthy pairs explains why this is the case. Because bonding electrons are sandwiched between two nuclei, they have far less space to "move about" than lone pairs. Between the nuclei of two atoms, a bond is basically an area of extremely high electron density. This means that the orbitals holding these bonding electrons are longer than those holding lone pairs. The orbitals that contain lone pairs are rounder and shorter in comparison to the orbitals that store bonding electrons. The orbitals in which lone pairs dwell are more spherical than the orbitals in which bonding electrons reside.
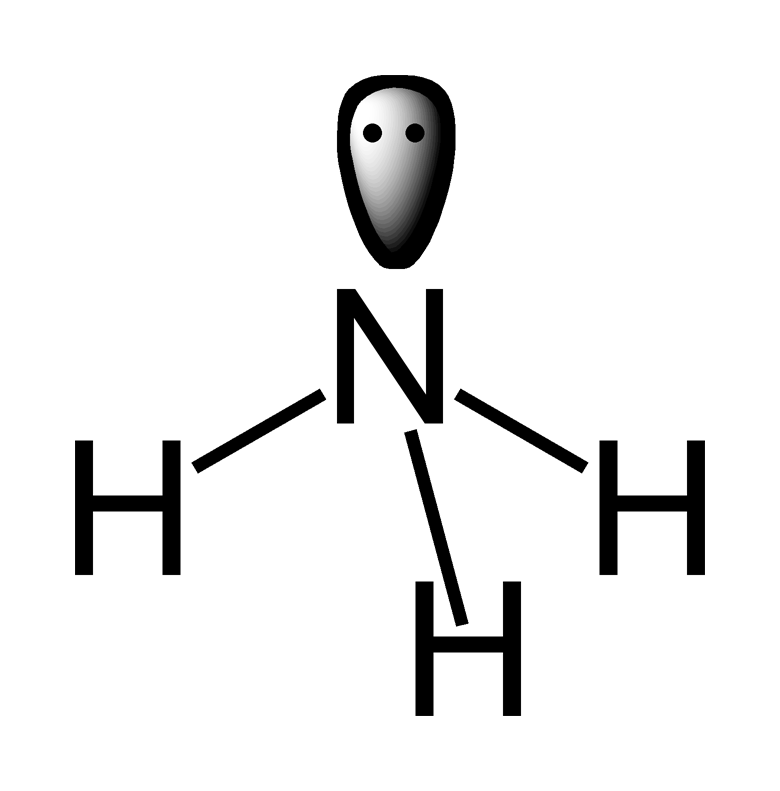
The lone pair of electrons on the ammonia molecule, AMMONIA, pulls down on the three bonds in this case. Because they are only attracted by one nucleus, lone pairs of electrons take up more space than bonded electrons because they are spread out at a shorter distance from that nucleus. Bonding electrons are further from the nucleus, but they are more confined, therefore they aren't as dispersed. This is why two lone pairs will repel each other more than one lone pair and one bond pair, which will repel each other more than two bond pairs.
Note:
A pair of lonely electrons, also known as an unshared or nonbonding pair, is a pair of electrons that are not shared in a covalent bond by another atom in chemistry. Atoms have lone pairs in their outermost electron shell. A Lewis structure can be used to identify them. When two electrons are linked but not utilised in chemical bonding, they are referred to as lone pairs.
Complete answer:
Because the electrons in a lone pair are not involved in bonding and are thus free, the repulsion is higher. Because of one free electron, the repulsion between a lone pair and a bond pair is somewhat higher than that between a bond pair and a bond pair. It's because the orbitals that house lone pairs have a distinct form. The repulsion between two lone pairs of electrons is, in fact, higher than the repulsion between a lone pair and a bonded pair of electrons. The repulsion between a lone pair of electrons and a bonding pair of electrons is also larger than the repulsion between two bonding pairs of electrons.
The geometry of the orbitals that hold these lengthy pairs explains why this is the case. Because bonding electrons are sandwiched between two nuclei, they have far less space to "move about" than lone pairs. Between the nuclei of two atoms, a bond is basically an area of extremely high electron density. This means that the orbitals holding these bonding electrons are longer than those holding lone pairs. The orbitals that contain lone pairs are rounder and shorter in comparison to the orbitals that store bonding electrons. The orbitals in which lone pairs dwell are more spherical than the orbitals in which bonding electrons reside.

The lone pair of electrons on the ammonia molecule, AMMONIA, pulls down on the three bonds in this case. Because they are only attracted by one nucleus, lone pairs of electrons take up more space than bonded electrons because they are spread out at a shorter distance from that nucleus. Bonding electrons are further from the nucleus, but they are more confined, therefore they aren't as dispersed. This is why two lone pairs will repel each other more than one lone pair and one bond pair, which will repel each other more than two bond pairs.
Note:
A pair of lonely electrons, also known as an unshared or nonbonding pair, is a pair of electrons that are not shared in a covalent bond by another atom in chemistry. Atoms have lone pairs in their outermost electron shell. A Lewis structure can be used to identify them. When two electrons are linked but not utilised in chemical bonding, they are referred to as lone pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




