
Though covalent in nature, methanol is soluble in water, why?
A) Methanol is transparent like water.
B) Due to hydrogen bonding between methanol and water molecules.
C) Due to van der Waals between methanol and water.
D) Due to covalent attraction forces.
Answer
567.3k+ views
Hint: A substance or solute dissolve in the solvent if the attraction between the solute particles and the solvent (water) is strong. It is strong enough to overcome the solute –solute and solvent –solvent forces of attraction and establishes a very strong interaction between solute and solvent molecules. Therefore methanol (lower alcohol) has good solubility in water.
Complete Solution :
- Solubility is a property of a solute to get dissolved in a solvent. We know that like dissolves like. According to which polar solute is dissolved in a polar solvent and nonpolar or covalent in a nonpolar solvent.
- Water is a polar solvent. It breaks down the polar solute into its ion and forms a solvent cage around the ion and dissolves the solute. However, it cannot be used to dissolve non-polar solvents like alcohol.
However, it is found that lower alcohol is highly soluble in water. The solubility of the alcohols in water is inversely related to the molecular weight. The solubility of alcohol decreases with an increase in molecular weight. The solubility of lower alcohols in water is due to the formation of a hydrogen bond between alcohols and the water molecules.
- The water molecules are polar in nature and hydrogen of one water molecule forms a hydrogen bond with the oxygen of another water molecule. The water is an extensive framework of hydrogen bonds.
- Methanol is a clear, colourless liquid. It is miscible in water. Water can easily form hydrogen bonds with the hydroxyl group of methanol. The hydrogen bonding is so strong that it is responsible for the solubility of the lower members of alcohol (like methanol, ethanol, or propanol) in water. The hydrogen bonding between the water and methanol is as follows:
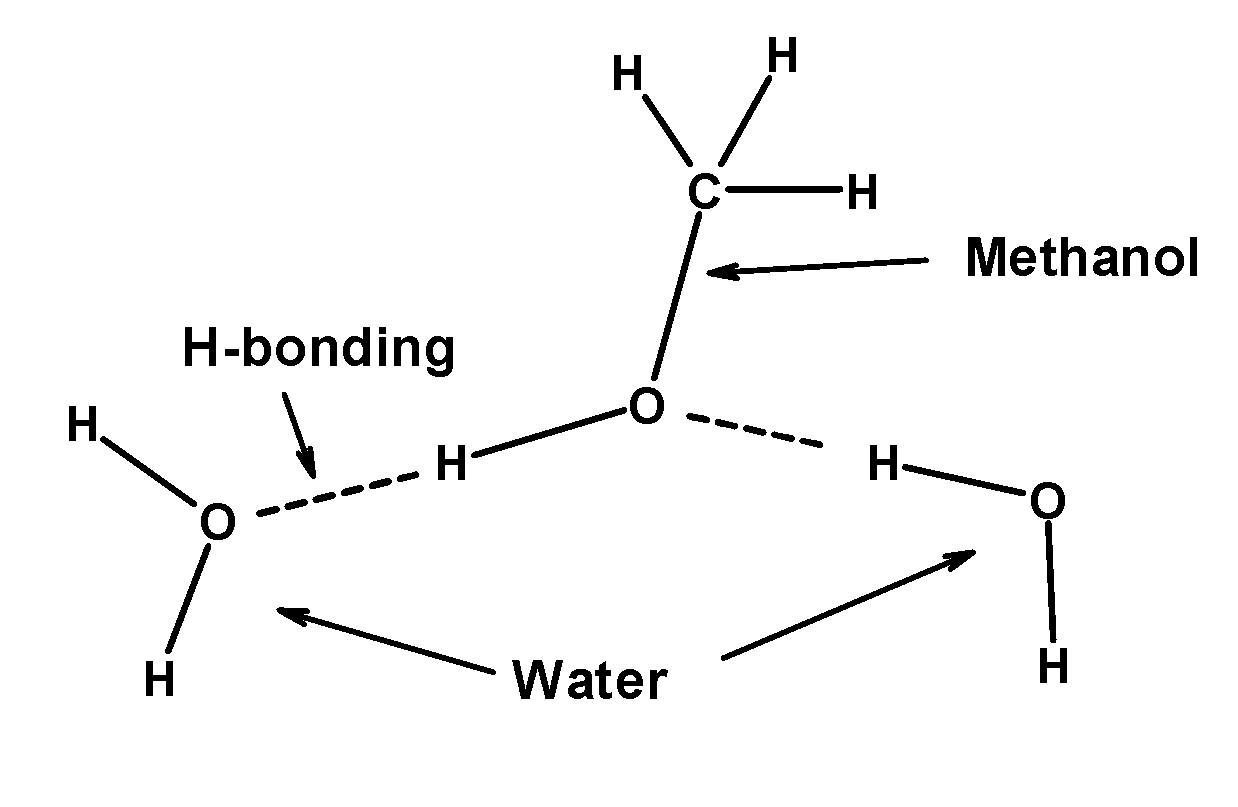
Therefore, the methanol is soluble in water due to hydrogen bonding between methanol and water molecules. So, the correct answer is “Option B”.
Note: Note that the water is a polar solvent and dissolves most of the polar solute by breaking them into its corresponding ions. However, the alcohol or organic molecules are soluble in water due to hydrogen bonds. It does not form ions in solution. It remains in its original state in the water framework and forms H-bonding.
Complete Solution :
- Solubility is a property of a solute to get dissolved in a solvent. We know that like dissolves like. According to which polar solute is dissolved in a polar solvent and nonpolar or covalent in a nonpolar solvent.
- Water is a polar solvent. It breaks down the polar solute into its ion and forms a solvent cage around the ion and dissolves the solute. However, it cannot be used to dissolve non-polar solvents like alcohol.
However, it is found that lower alcohol is highly soluble in water. The solubility of the alcohols in water is inversely related to the molecular weight. The solubility of alcohol decreases with an increase in molecular weight. The solubility of lower alcohols in water is due to the formation of a hydrogen bond between alcohols and the water molecules.
- The water molecules are polar in nature and hydrogen of one water molecule forms a hydrogen bond with the oxygen of another water molecule. The water is an extensive framework of hydrogen bonds.
- Methanol is a clear, colourless liquid. It is miscible in water. Water can easily form hydrogen bonds with the hydroxyl group of methanol. The hydrogen bonding is so strong that it is responsible for the solubility of the lower members of alcohol (like methanol, ethanol, or propanol) in water. The hydrogen bonding between the water and methanol is as follows:

Therefore, the methanol is soluble in water due to hydrogen bonding between methanol and water molecules. So, the correct answer is “Option B”.
Note: Note that the water is a polar solvent and dissolves most of the polar solute by breaking them into its corresponding ions. However, the alcohol or organic molecules are soluble in water due to hydrogen bonds. It does not form ions in solution. It remains in its original state in the water framework and forms H-bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




