
Total number of hydroxyl groups present in a molecule of the major product R is _________.
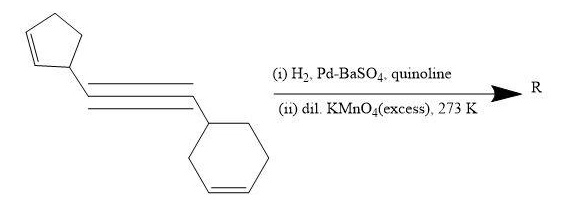
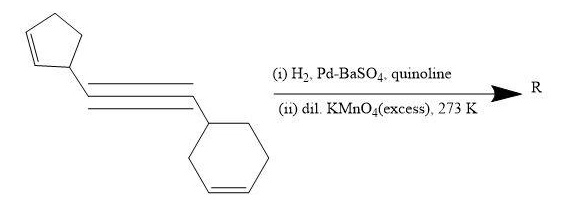
Answer
578.4k+ views
Hint: The number of hydroxyl groups in the product symbolises the presence of -OH group to the other atoms in the molecule.
Presence of double bond in the reactant goes for attachment of two hydroxyl groups each when the product is formed.
Complete step by step solution:
The given reaction is about reduction and oxidation one after the other using a specific catalyst which would make the reaction possible at room temperature.
Step I- Reduction of triple bond.
A Lindlar catalyst is a heterogeneous catalyst which consists of palladium deposited on barium sulphate. This catalyst is used for the hydrogenation of alkynes to cis-alkenes, without further reduction to alkanes.
When a triple bond is hydrogenated over Lindlar’s catalyst ($Pd/BaS{{O}_{4}}$) it gives cis-alkene predominantly.
Step II- Oxidation of double bond.
Oxidation of alkenes takes place when solution of potassium permanganate is added in cold conditions. The electrophilic addition mechanism takes place in the reaction.
Here, $KMn{{O}_{4}}$ gets reduced while it oxidises the alkene group. The electrons are being transferred, resulting in O atoms being in the form of alcohols. These O atoms who became alcohols will be on same side of the ring and hence, be cis.
Reaction for the given illustration-
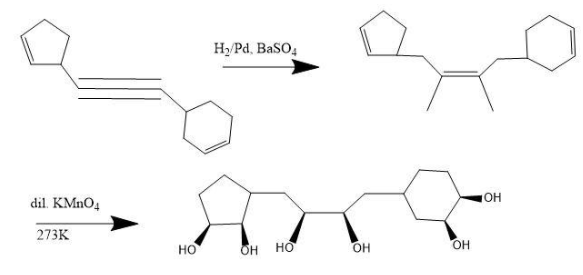
Here, we can see that each double bond has resulted in two hydroxyl groups being attached to the chain at the end.
Firstly, triple bond reduces to double bond and then the oxidation results into formation of alcohol substitutes.
Therefore, the total number of hydroxyl groups attached to the major product are 6.
Note: Do note that the cis-alkene is formed when we reduce alkyne with the use of Lindlar’s catalyst. This catalyst prevents the formation of alkane by further reduction.
And potassium permanganate reacted to the alkene in cold conditions will give this product. If conditions are different, the mechanism will change.
Presence of double bond in the reactant goes for attachment of two hydroxyl groups each when the product is formed.
Complete step by step solution:
The given reaction is about reduction and oxidation one after the other using a specific catalyst which would make the reaction possible at room temperature.
Step I- Reduction of triple bond.
A Lindlar catalyst is a heterogeneous catalyst which consists of palladium deposited on barium sulphate. This catalyst is used for the hydrogenation of alkynes to cis-alkenes, without further reduction to alkanes.
When a triple bond is hydrogenated over Lindlar’s catalyst ($Pd/BaS{{O}_{4}}$) it gives cis-alkene predominantly.
Step II- Oxidation of double bond.
Oxidation of alkenes takes place when solution of potassium permanganate is added in cold conditions. The electrophilic addition mechanism takes place in the reaction.
Here, $KMn{{O}_{4}}$ gets reduced while it oxidises the alkene group. The electrons are being transferred, resulting in O atoms being in the form of alcohols. These O atoms who became alcohols will be on same side of the ring and hence, be cis.
Reaction for the given illustration-

Here, we can see that each double bond has resulted in two hydroxyl groups being attached to the chain at the end.
Firstly, triple bond reduces to double bond and then the oxidation results into formation of alcohol substitutes.
Therefore, the total number of hydroxyl groups attached to the major product are 6.
Note: Do note that the cis-alkene is formed when we reduce alkyne with the use of Lindlar’s catalyst. This catalyst prevents the formation of alkane by further reduction.
And potassium permanganate reacted to the alkene in cold conditions will give this product. If conditions are different, the mechanism will change.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




