
What is the total number of p electrons in a single phosphorus atom in its ground state?
A. 3
B. 5
C. 9
D. 15
Answer
578.7k+ views
Hint: Phosphorus is a p-block element. Because the last electron enters in the p-orbital. Velocity is calculated by total numbers of electrons and is calculated by counting numbers of unpaired electrons present in orbitals.
Complete step by step answer:
Phosphorus element belongs to group 15
Atomic number of phosphorus is 15
Its electronic configuration is 2, 8, 5 or \[1{s^2},{\text{ }}2{s^2},{\text{ }}2{p^6},{\text{ }}3{s^2},{\text{ }}3{p^3}\]
If this configuration is represented in the form of box, we have
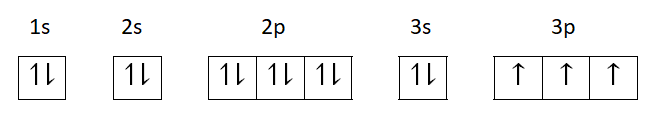
Here, we see that the total number of electrons in s-orbital is 9
2p orbital has 6 electrons and 3p orbital has 3 electrons.
Therefore, total electrons are $6 + 3 = 9$electrons.
Electron filling takes place in an orbital according to aufbau principle. Electrons occupy the orbitals according to increasing energy levels. Energy level of 1s orbital is least so electrons enter in 1s orbital first. Energy level of orbitals is as follows.
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p > 5s and so on
It also obeys Hund’s rule of maximum multiplicity.
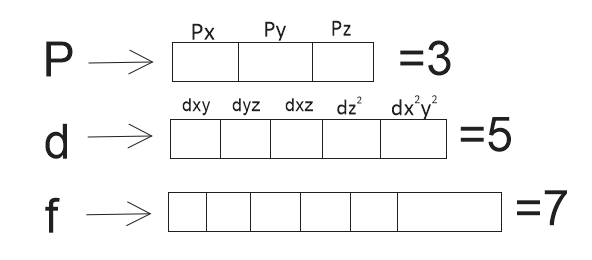
According to this rule electron pairing in p, d and f orbitals cannot occur until each orbital of given subshell contains one electron each or singly occupied subshell in p, d and f are as follows
Note:
White filling electrons in orbitals should obey aufbau principle and Hund’s rule of maximum multiplicity while counting no of electrons in different orbitals. Be careful to note that 4s is filled before 3d.
Complete step by step answer:
Phosphorus element belongs to group 15
Atomic number of phosphorus is 15
Its electronic configuration is 2, 8, 5 or \[1{s^2},{\text{ }}2{s^2},{\text{ }}2{p^6},{\text{ }}3{s^2},{\text{ }}3{p^3}\]
If this configuration is represented in the form of box, we have

Here, we see that the total number of electrons in s-orbital is 9
2p orbital has 6 electrons and 3p orbital has 3 electrons.
Therefore, total electrons are $6 + 3 = 9$electrons.
Electron filling takes place in an orbital according to aufbau principle. Electrons occupy the orbitals according to increasing energy levels. Energy level of 1s orbital is least so electrons enter in 1s orbital first. Energy level of orbitals is as follows.
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p > 5s and so on
It also obeys Hund’s rule of maximum multiplicity.
According to this rule electron pairing in p, d and f orbitals cannot occur until each orbital of given subshell contains one electron each or singly occupied subshell in p, d and f are as follows

Note:
White filling electrons in orbitals should obey aufbau principle and Hund’s rule of maximum multiplicity while counting no of electrons in different orbitals. Be careful to note that 4s is filled before 3d.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




