
When trans-2-butene is reacted with $B{{r}_{2}}$ then product is formed:
A. Racemic-2,3-dibromobutane
B. Meso-2,3-dibromobutane
C. d-2,3-dibromobutane
D. l-2,3-dibromobutane
Answer
515.4k+ views
Hint: Alkenes generally undergo additional chemical reactions with other chemicals due to the presence of a high number of electrons with them. The formation of the major product is going to depend on the presence of the substituents in the alkenes.
Complete answer:
- In the question it is asked to find the product when trans-2-butene is going to react with bromine among the given options.
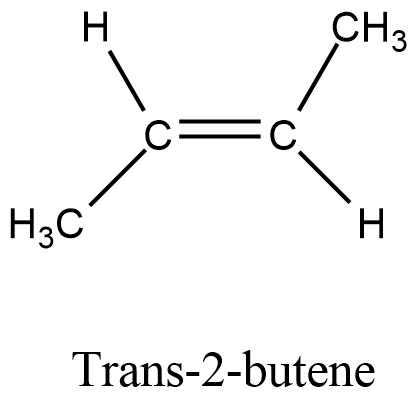
- First, we should know the structure of the trans-2-butene and it is as follows.
- Now, we have to write the chemical reaction between trans-2-butene and bromine and it is as follows.
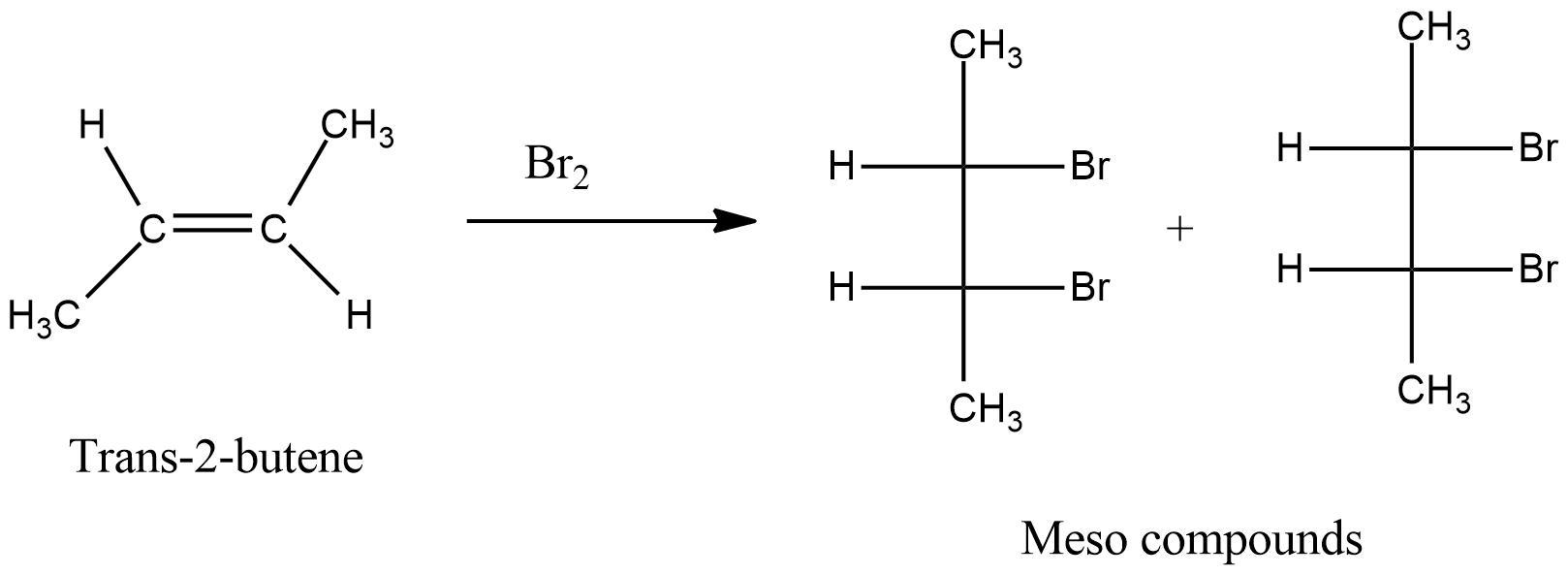
- In the above chemical reaction, we can see that the bromine is going to react on the double bonded carbon in two different directions.
- When the bromine is going to react with double bonded carbon from the above side and from the below side the formed products are d and l-form.
- Means we are going to get a meso compound when bromine is going to react with trans-2-butene.
Therefore, the correct option is B, Meso-2,3-dibromo butane.
Note:
The formation of the products in the additional chemical reactions on the highly substituted alkenes is going to depend on the reagent which is going to be involved in the chemical reaction. If the reagent is homodimer, then the product will be meso in nature.
Complete answer:
- In the question it is asked to find the product when trans-2-butene is going to react with bromine among the given options.
- First, we should know the structure of the trans-2-butene and it is as follows.

- Now, we have to write the chemical reaction between trans-2-butene and bromine and it is as follows.

- In the above chemical reaction, we can see that the bromine is going to react on the double bonded carbon in two different directions.
- When the bromine is going to react with double bonded carbon from the above side and from the below side the formed products are d and l-form.
- Means we are going to get a meso compound when bromine is going to react with trans-2-butene.
Therefore, the correct option is B, Meso-2,3-dibromo butane.
Note:
The formation of the products in the additional chemical reactions on the highly substituted alkenes is going to depend on the reagent which is going to be involved in the chemical reaction. If the reagent is homodimer, then the product will be meso in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




