
Triple point of water is
A. $373.16\text{K}$
B. ${\text{373}}{\text{.16}}{{\text{ }}^ \circ }{\text{F}}$
C. $273.16\text{K}$
D. ${\text{273}}{\text{.16}}{{\text{ }}^ \circ }{\text{F}}$
Answer
588k+ views
Hint: Triple point is a point where all the three states exist in equilibrium with each other. It is always a fixed point and also used in thermometer calibrations.
Complete step by step answer:
Triple point is a point with a particular pressure and temperature value at which all the three States of matter that is solid, liquid and gas exist in equilibrium with each other. There are three curves related to water, sublimation curve, fusion curve and vaporization curve.
Sublimation curve is the curve at which the solid state and the gas states are in equilibrium with each other fusion curve is the curve when liquid state and solid state are in equilibrium with each other and vaporization curve is that in which liquid state and the gaseous state are in equilibrium with each other. At the Triple point sublimation curve, the fusion curve and the vaporization ka meet each other at a common point.
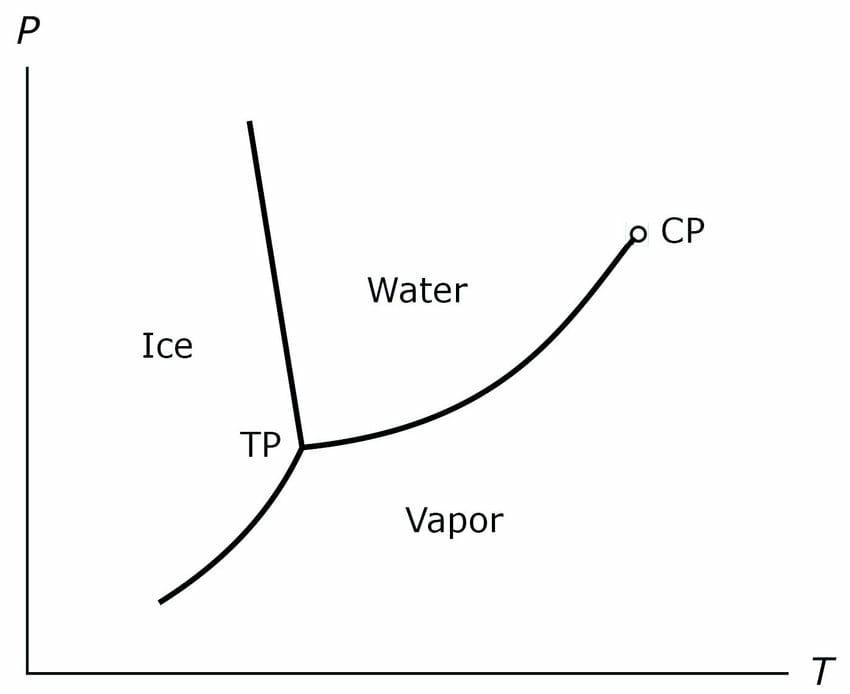
That is why the name is triple point because the 3 stages meet each other or existing equilibrium. Equilibrium is a state where the reactant and product keeps on inter converting into each other. For example in solid liquid equilibrium, solid water gets converted into liquid water and the liquid water will convert into solid water, simultaneously, so that the concentration of both solid water and the liquid water will remain constant.
The value of temperature and pressure at which this phenomenon occur is:
Temperature: 273.16 K
Pressure: 611.657 pascal
Hence, the correct option is C .
Note:
Critical point is the end point of the curve (of any phase equilibrium curve). At critical point liquid and vapor coexist. Most commonly we use to remember the temperature in Kelvin scale but sometimes it can even ask in degree Celsius or Fahrenheit scale for that we can do the following conversion:
${\text{T(in kelvin) = T}}{{\text{(}}^ \circ }{\text{C) + 273}}$
${\text{T }}{{\text{(}}^ \circ }{\text{F) = 1}}{{.8 \times (T(in K) - 273) + 32}}$
Complete step by step answer:
Triple point is a point with a particular pressure and temperature value at which all the three States of matter that is solid, liquid and gas exist in equilibrium with each other. There are three curves related to water, sublimation curve, fusion curve and vaporization curve.
Sublimation curve is the curve at which the solid state and the gas states are in equilibrium with each other fusion curve is the curve when liquid state and solid state are in equilibrium with each other and vaporization curve is that in which liquid state and the gaseous state are in equilibrium with each other. At the Triple point sublimation curve, the fusion curve and the vaporization ka meet each other at a common point.

That is why the name is triple point because the 3 stages meet each other or existing equilibrium. Equilibrium is a state where the reactant and product keeps on inter converting into each other. For example in solid liquid equilibrium, solid water gets converted into liquid water and the liquid water will convert into solid water, simultaneously, so that the concentration of both solid water and the liquid water will remain constant.
The value of temperature and pressure at which this phenomenon occur is:
Temperature: 273.16 K
Pressure: 611.657 pascal
Hence, the correct option is C .
Note:
Critical point is the end point of the curve (of any phase equilibrium curve). At critical point liquid and vapor coexist. Most commonly we use to remember the temperature in Kelvin scale but sometimes it can even ask in degree Celsius or Fahrenheit scale for that we can do the following conversion:
${\text{T(in kelvin) = T}}{{\text{(}}^ \circ }{\text{C) + 273}}$
${\text{T }}{{\text{(}}^ \circ }{\text{F) = 1}}{{.8 \times (T(in K) - 273) + 32}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




