
What is true about a simple cubic type of unit cell?
(A) Eight constituents are at different corners of the cube.
(B) The number of atoms per unit cell is 1.
(C) Contribution by an atom at one corner is $ \dfrac{1}{8}\text{th} $ of an atom.
(D) None of the above.
Answer
555k+ views
Hint: In simple cubic type unit cell 1 atom is used by eight corners of a cube. Four unit cells are present at the upper and lower layer of the cube $ 8\times \dfrac{1}{8}=1 $ .
Complete Step by step solution
In the primitive cubic unit cell, the atoms are present only at the corners. Every atom at the corner is shared among 8 adjacent unit cells. There are 4 unit cells in the same layer and 4 in the upper (or lower) layer. Therefore, a particular unit cell has the only $ \dfrac{1}{8}\text{th} $ of an atom.
Some point of simple cubic unit cell are-
The atoms in the primitive cubic unit cell are present only at the corners
Every atom at the corner is shared among eight adjacent unit cells
Four unit cells are present in the same layer
Four unit cell in the upper/lower layer
Therefore, a particular unit cell has the only 1/8th of an atom
Each small sphere in the following figure represents the centre of a particle which occupies that particular position and not its size
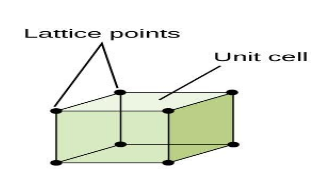
In each cubic unit cell, there are 8 atoms at the corners. Therefore, the total number of atoms in one unit cell is
$ 8\times \dfrac{1}{8}=1 $ atom.
Hence B is correct option.
Additional information
Unit cells are the basic structure of a crystal. Different crystals have different structures. 3 types of unit cell are simple cubic unit cell, body centred cubic unit cell and face centered cubic unit cell. In a body centered cubic there are eight identical particles on the eight corners of a unit cell and ninth identical particle in the center of the body of the unit cell.
Note
Different crystals have different structures and different properties make sure you are clear about them. Understand the distribution of atoms in a unit cell and their shape. The face-centered cubic unit cell also starts with identical particles on the eight corners of the cube. But this structure also contains the same particles in the centers of the six faces of the unit cell, for a total of 14 identical lattice points.
Complete Step by step solution
In the primitive cubic unit cell, the atoms are present only at the corners. Every atom at the corner is shared among 8 adjacent unit cells. There are 4 unit cells in the same layer and 4 in the upper (or lower) layer. Therefore, a particular unit cell has the only $ \dfrac{1}{8}\text{th} $ of an atom.
Some point of simple cubic unit cell are-
The atoms in the primitive cubic unit cell are present only at the corners
Every atom at the corner is shared among eight adjacent unit cells
Four unit cells are present in the same layer
Four unit cell in the upper/lower layer
Therefore, a particular unit cell has the only 1/8th of an atom
Each small sphere in the following figure represents the centre of a particle which occupies that particular position and not its size

In each cubic unit cell, there are 8 atoms at the corners. Therefore, the total number of atoms in one unit cell is
$ 8\times \dfrac{1}{8}=1 $ atom.
Hence B is correct option.
Additional information
Unit cells are the basic structure of a crystal. Different crystals have different structures. 3 types of unit cell are simple cubic unit cell, body centred cubic unit cell and face centered cubic unit cell. In a body centered cubic there are eight identical particles on the eight corners of a unit cell and ninth identical particle in the center of the body of the unit cell.
Note
Different crystals have different structures and different properties make sure you are clear about them. Understand the distribution of atoms in a unit cell and their shape. The face-centered cubic unit cell also starts with identical particles on the eight corners of the cube. But this structure also contains the same particles in the centers of the six faces of the unit cell, for a total of 14 identical lattice points.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




