
What is true of membrane lipids and proteins?
A. None can flip-flop
B. Both can flip-flop
C. Proteins can flip-flop but lipids cannot.
D. Lipids flip-flop but proteins rarely flip-flop
Answer
565.2k+ views
Hint:A thin polar membrane is formed from two layers of lipid molecules in the lipid bilayer (or phospholipid bilayer). Amphiphilic phospholipids with a hydrophilic phosphate head and a hydrophobic tail consisting of two fatty acid chains are normally composed of biological bilayers.
Complete answer:Phospholipids, arranged in a bilayer, make up the plasma membrane’s essential cloth. Since they are amphipathic, they are well-suited for this function, meaning they have both hydrophilic and hydrophobic regions.
The hydrophilic or "water-loving" component of a phospholipid is its head, which includes a phosphate group that is negatively charged, as well as an additional small group that can also be charged or polar.
Its long, nonpolar fatty acid tails make up the hydrophobic, or "water-fearing," portion of a phospholipid. The tails of fatty acids can interact easily with other non-polar molecules but interact poorly with water. Because of this, tucking their fatty acid tails away into the interior of the membrane, where they are protected from the ambient water, is more energetically advantageous for the phospholipids.
As their name means, integral membrane proteins are inserted into the membrane: they have at least one hydrophobic region that anchors them to the phospholipid bilayer's hydrophobic center. Others only stick to the membrane slightly, while others extend from one side of the membrane to the other and are exposed on either side of the membrane.
Below is the picture of the phospholipid bilayer.
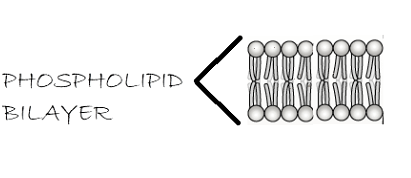
Hence the correct answer is option D, Lipids flip-flop but proteins rarely flip-flop.
Note: Hydrophilic head groups faced outwards and hydrophobic tails faced each other is part of the lipid bilayer of the cell membrane. Owing to this bipolar design, the transportation of phospholipids and lipid-linked oligosaccharides is difficult to accomplish. The proteins of the transmembrane lipid transporter called flippases transport lipids by transverse diffusion by flipping their direction around the cell membrane.
Reverse path transport of floppases. This influences multiple mechanisms in animals, such as blood coagulation, immune recognition, and programmed death of cells. Owing to their extensive polar areas, which are undesirable in the hydrophobic center of the membrane, most protein molecules do not flip flop.
Complete answer:Phospholipids, arranged in a bilayer, make up the plasma membrane’s essential cloth. Since they are amphipathic, they are well-suited for this function, meaning they have both hydrophilic and hydrophobic regions.
The hydrophilic or "water-loving" component of a phospholipid is its head, which includes a phosphate group that is negatively charged, as well as an additional small group that can also be charged or polar.
Its long, nonpolar fatty acid tails make up the hydrophobic, or "water-fearing," portion of a phospholipid. The tails of fatty acids can interact easily with other non-polar molecules but interact poorly with water. Because of this, tucking their fatty acid tails away into the interior of the membrane, where they are protected from the ambient water, is more energetically advantageous for the phospholipids.
As their name means, integral membrane proteins are inserted into the membrane: they have at least one hydrophobic region that anchors them to the phospholipid bilayer's hydrophobic center. Others only stick to the membrane slightly, while others extend from one side of the membrane to the other and are exposed on either side of the membrane.
Below is the picture of the phospholipid bilayer.

Hence the correct answer is option D, Lipids flip-flop but proteins rarely flip-flop.
Note: Hydrophilic head groups faced outwards and hydrophobic tails faced each other is part of the lipid bilayer of the cell membrane. Owing to this bipolar design, the transportation of phospholipids and lipid-linked oligosaccharides is difficult to accomplish. The proteins of the transmembrane lipid transporter called flippases transport lipids by transverse diffusion by flipping their direction around the cell membrane.
Reverse path transport of floppases. This influences multiple mechanisms in animals, such as blood coagulation, immune recognition, and programmed death of cells. Owing to their extensive polar areas, which are undesirable in the hydrophobic center of the membrane, most protein molecules do not flip flop.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




