
Two metals which will replace hydrogen and two metals which will not displace hydrogen from dilute acid, respectively are:
A. ${\text{Potassium ,calcium ,aluminium and zinc}}$
B. ${\text{Sodium, calcium, zinc and iron}}$
C. ${\text{Zinc, iron, copper and mercury}}$
D. ${\text{Copper, mercury, Silver and gold}}$
Answer
539.4k+ views
Hint: Judge the reactivity on the basis of Reactivity Series table as in this case 2 metals are above hydrogen and 2 metals are below hydrogen in reactivity series.
Only those metals will replace hydrogen from dilute acid which are above hydrogen in reactivity series
And , hydrogen will not be replaced by those metals which are below hydrogen in reactivity series.
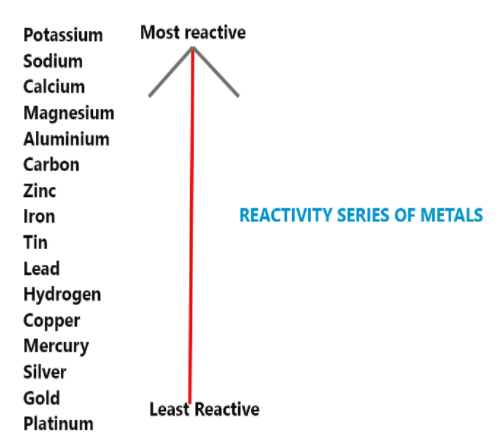
Hence,Option C is the correct answer.
Additional Information:
In the reactivity series, as we move from bottom to top, the reactivity of metals increases. Metals present at the top of the series can lose electrons more readily to form positive ions and corrode or tarnish more readily. They require more energy to be separated from their ores, and become stronger reducing agents, while metals present at the bottom of the series are good oxidizing agents.
Silver, gold, and platinum are metals with the least reactivity. They are found in nature. The metals with high reactive series in the above table also indicate that reverse reaction is a bit tough process.
Reactions are mostly Exothermic in case of highly reactive metals.
The reaction takes place fast in highly reactive metals.
Metals which are present above the carbon are extracted using an electrolysis method.
Carbon and hydrogen plays a vital role in terms of a method that involves metal extraction.
Note:
1) Students should ensure they keep themselves in check with the reactivity series.
2) Reactivity series is different for non-metals.
Only those metals will replace hydrogen from dilute acid which are above hydrogen in reactivity series
And , hydrogen will not be replaced by those metals which are below hydrogen in reactivity series.

Hence,Option C is the correct answer.
Additional Information:
In the reactivity series, as we move from bottom to top, the reactivity of metals increases. Metals present at the top of the series can lose electrons more readily to form positive ions and corrode or tarnish more readily. They require more energy to be separated from their ores, and become stronger reducing agents, while metals present at the bottom of the series are good oxidizing agents.
Silver, gold, and platinum are metals with the least reactivity. They are found in nature. The metals with high reactive series in the above table also indicate that reverse reaction is a bit tough process.
Reactions are mostly Exothermic in case of highly reactive metals.
The reaction takes place fast in highly reactive metals.
Metals which are present above the carbon are extracted using an electrolysis method.
Carbon and hydrogen plays a vital role in terms of a method that involves metal extraction.
Note:
1) Students should ensure they keep themselves in check with the reactivity series.
2) Reactivity series is different for non-metals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




