
Two systems are in thermal equilibrium. The quantity which is common for them is
A. Heat
B. Momentum
C. specific heat
D. temperature
Answer
585.9k+ views
Hint: Zeroth law of thermodynamics states that two systems A and B, which are separately in thermal equilibrium with a third system C, are also in thermal equilibrium with each other. Any isolated system is in thermodynamic equilibrium.
Complete step by step answer:
Consider two systems A and B, separated by a wall that does not allow any exchange of energy between them. Such a wall is known as an insulating wall or adiabatic wall. The third system C is separated from system A and B by a conducting or diathermic wall.
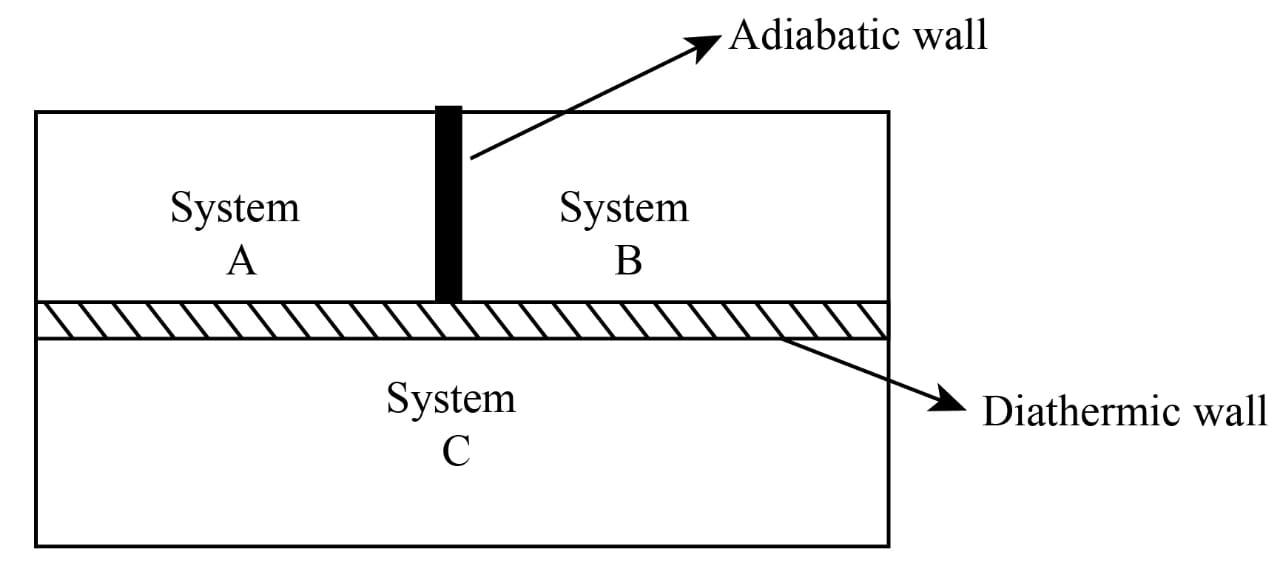
Since energy can be exchanged between the system A and C, so both system A and C are in thermal equilibrium. Similarly, energy can be exchanged between systems B and C. So both B and C are in thermal equilibrium. When the adiabatic wall between the systems A and B are removed, no energy transfer can take place between them because the temperature is the same; this shows that the two systems A and B are in thermal equilibrium; this observation leads to an important law known as Zeroth law of thermodynamics.
Hence, the correct option is (D).
Note:
Concept of temperature from Zeroth law of thermodynamics:
According to law, if system A is in thermal equilibrium with a system C,
Then the temperature of system A = Temperature of system C
Similarly, if system B is in thermal equilibrium with system C,
The temperature of system B = temperature of system C
Now, from the above relation, we conclude that,
The temperature of system A = Temperature of system B
Therefore, the temperature of a system is a physical quantity, equality of which is the only condition for thermal equilibrium.
Complete step by step answer:
Consider two systems A and B, separated by a wall that does not allow any exchange of energy between them. Such a wall is known as an insulating wall or adiabatic wall. The third system C is separated from system A and B by a conducting or diathermic wall.

Since energy can be exchanged between the system A and C, so both system A and C are in thermal equilibrium. Similarly, energy can be exchanged between systems B and C. So both B and C are in thermal equilibrium. When the adiabatic wall between the systems A and B are removed, no energy transfer can take place between them because the temperature is the same; this shows that the two systems A and B are in thermal equilibrium; this observation leads to an important law known as Zeroth law of thermodynamics.
Hence, the correct option is (D).
Note:
Concept of temperature from Zeroth law of thermodynamics:
According to law, if system A is in thermal equilibrium with a system C,
Then the temperature of system A = Temperature of system C
Similarly, if system B is in thermal equilibrium with system C,
The temperature of system B = temperature of system C
Now, from the above relation, we conclude that,
The temperature of system A = Temperature of system B
Therefore, the temperature of a system is a physical quantity, equality of which is the only condition for thermal equilibrium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




