
What type of rings present in rhombic sulphur?
A)${{S}_{8}}$chains
B) ${{S}_{2}}$molecules
C) ${{S}_{4}}$rings
D) ${{S}_{8}}$rings
Answer
538.5k+ views
Hint:The answer lies in the fact about rhombic sulphur that it is the yellow coloured compound also called as$\alpha $- sulphur where it has the thermal stability and its allotropes are interconvertible and this when heated above 369 K gives the monoclinic sulphur. Based on these facts draw the structure of rhombic sulphur which gives the answer.
Complete step-by-step answer:The concept of general chemistry that tells about the crystal lattices and their properties ad structures are familiar to us.
We shall now see the structure of rhombic sulphur based on the facts regarding the rhombic sulphur.
- Rhombic sulphur is the crystalline allotropic form of sulphur
- The existence of${{S}_{2}}$molecules is not possible because of the low enthalpy of the$S-S$bond.
- In case of option C)${{S}_{4}}$rings of sulphur will have greater angle strain and is also unstable at room temperature.
- Sulphur does not show any catenation and therefore the valency of the sulphur in rhombic form is high so that it does not form chains and thus option A) is ruled out.
- The stable form of sulphur that exists at room temperature that is below 369 K is the rhombohedral which exists in the form of a ring with 8 sulphur atoms. Thus it forms the rings and the structure is as shown below,
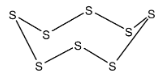
Thus, option D) is the correct answer
Note:Note that rhombic sulphur is the most stable allotrope and is crystalline in nature and all the other varieties of sulphur eventually revert to rhombic form on standing and this when slowly heated to about 359 K it changes to monoclinic or$\beta $- sulphur and when cooled below same temperature it changes back to rhombic form.
Complete step-by-step answer:The concept of general chemistry that tells about the crystal lattices and their properties ad structures are familiar to us.
We shall now see the structure of rhombic sulphur based on the facts regarding the rhombic sulphur.
- Rhombic sulphur is the crystalline allotropic form of sulphur
- The existence of${{S}_{2}}$molecules is not possible because of the low enthalpy of the$S-S$bond.
- In case of option C)${{S}_{4}}$rings of sulphur will have greater angle strain and is also unstable at room temperature.
- Sulphur does not show any catenation and therefore the valency of the sulphur in rhombic form is high so that it does not form chains and thus option A) is ruled out.
- The stable form of sulphur that exists at room temperature that is below 369 K is the rhombohedral which exists in the form of a ring with 8 sulphur atoms. Thus it forms the rings and the structure is as shown below,

Thus, option D) is the correct answer
Note:Note that rhombic sulphur is the most stable allotrope and is crystalline in nature and all the other varieties of sulphur eventually revert to rhombic form on standing and this when slowly heated to about 359 K it changes to monoclinic or$\beta $- sulphur and when cooled below same temperature it changes back to rhombic form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




