
What type of water is least dense?
Answer
524.7k+ views
Hint: The density is the amount of mass present in specific volume of a substance. It depends upon the structural arrangement of the substance. The substance in which molecules and atoms are far apart from each other is said to be less dense.
\[\text{Density}=\dfrac{\text{Mass (g)}}{\text{Volume (c}{{\text{m}}^{3}})}\]
Complete answer:
Water is an essential part of our lives. It covers up to 70% of the earth’s surface. It exists in solid, liquid and gaseous phase within a low range of temperature and pressure.
Each water molecule is made up of two hydrogen atoms and one oxygen atom. These molecules of water are bonded to each other by hydrogen bonds in liquid state. Density is defined as the mass per unit volume of a substance. The density of water at room temperature is approximately $1\text{ g c}{{\text{m}}^{-3}}$. But the density of water changes with the change in temperature.
When water gradually cools, its mass remains constant but volume decreases. Since volume is inversely proportional to the density, thus density of water should increase with decrease in temperature. But when the temperature of water reaches $0{}^\circ \text{C}$, its density gets decreased.
At $0{}^\circ \text{C}$, water starts to freeze and forms ice. Ice has a rigid open lattice, web-like structure. The molecules in ice are arranged in a regular manner held by hydrogen bonds that form a crystalline lattice. These crystals have a number of vacant spaces that make ice less dense than water.
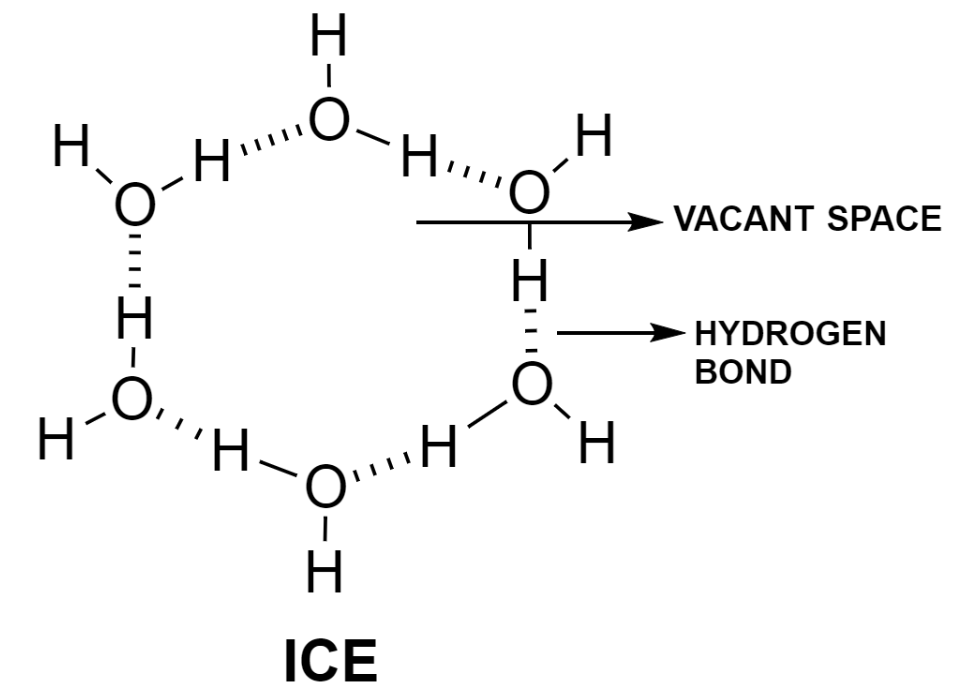
The density of water is maximum at $4{}^\circ \text{C}$ and it is equal to $1.0000\text{ g c}{{\text{m}}^{-3}}$ and after that density decreases and reaches a minimum value of $0.9168\text{ g c}{{\text{m}}^{-3}}$ at its freezing point $\left( 0{}^\circ \text{C} \right)$.
Hence, water at $0{}^\circ \text{C}$ (ice) is least dense.
Note:
The substance with lower value of density tends to float upon the substance with higher density. Since the density of water at$0{}^\circ \text{C}$ is less than the density of water at room temperature, this makes the water at $0{}^\circ \text{C}$, that is ice, float upon the liquid water. This is also why icebergs and ice cubes float on the surface of liquid water.
\[\text{Density}=\dfrac{\text{Mass (g)}}{\text{Volume (c}{{\text{m}}^{3}})}\]
Complete answer:
Water is an essential part of our lives. It covers up to 70% of the earth’s surface. It exists in solid, liquid and gaseous phase within a low range of temperature and pressure.
Each water molecule is made up of two hydrogen atoms and one oxygen atom. These molecules of water are bonded to each other by hydrogen bonds in liquid state. Density is defined as the mass per unit volume of a substance. The density of water at room temperature is approximately $1\text{ g c}{{\text{m}}^{-3}}$. But the density of water changes with the change in temperature.
When water gradually cools, its mass remains constant but volume decreases. Since volume is inversely proportional to the density, thus density of water should increase with decrease in temperature. But when the temperature of water reaches $0{}^\circ \text{C}$, its density gets decreased.
At $0{}^\circ \text{C}$, water starts to freeze and forms ice. Ice has a rigid open lattice, web-like structure. The molecules in ice are arranged in a regular manner held by hydrogen bonds that form a crystalline lattice. These crystals have a number of vacant spaces that make ice less dense than water.

The density of water is maximum at $4{}^\circ \text{C}$ and it is equal to $1.0000\text{ g c}{{\text{m}}^{-3}}$ and after that density decreases and reaches a minimum value of $0.9168\text{ g c}{{\text{m}}^{-3}}$ at its freezing point $\left( 0{}^\circ \text{C} \right)$.
Hence, water at $0{}^\circ \text{C}$ (ice) is least dense.
Note:
The substance with lower value of density tends to float upon the substance with higher density. Since the density of water at$0{}^\circ \text{C}$ is less than the density of water at room temperature, this makes the water at $0{}^\circ \text{C}$, that is ice, float upon the liquid water. This is also why icebergs and ice cubes float on the surface of liquid water.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




