
What do you understand by a lone pair of electrons? Draw the electron dot diagram of the Hydronium ion. $\left( \text{H}=1;\text{ O}=8 \right)$
Answer
533.7k+ views
Hint: To draw the electron dot diagram of hydronium ion, we need to find the number of valence electrons that take part in bonding and the number of unshared pairs of electrons in the outermost shell of each atom.
Complete answer:
Every element has a specific number of electrons present in its atomic orbitals. Out of these electrons, only those participating in bond formation with another atom which is present in the outermost shell of the atom. These electrons which are present in the outermost orbital of an atom are called valence electrons.
Any atom forms a bond with another atom to attain stability by completing its octet. And it is not necessary that each electron present in the valence shell takes part in octet formation. In some cases, it has been observed that one or more pairs of electrons in the valence shell remain unshared during bond formation. Such pairs of electrons in the outermost shell of an atom that remains unshared are known as lone pairs of electrons.
Hydronium ion is made by the addition of an ${{\text{H}}^{+}}$ ion to a water molecule. To draw the electron dot or Lewis diagram of hydronium ion, we first need to write the electronic configuration.
\[\begin{align}
& \text{H}-1{{\text{s}}^{1}} \\
& \text{O}-1{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{4}} \\
\end{align}\]
The oxygen has a total of 6 electrons in its valence shell out of which two electrons form a covalent bond with two hydrogen atoms and form a water molecule while the remaining two pairs of electrons exist as lone pairs of oxygen.

Now, this water molecule has two lone pairs of electrons. When an ${{\text{H}}^{+}}$ ion is added to a water molecule, one of the lone pairs of electrons is transferred from oxygen to hydrogen ion, and the formation of a coordinate bond takes place as shown below.
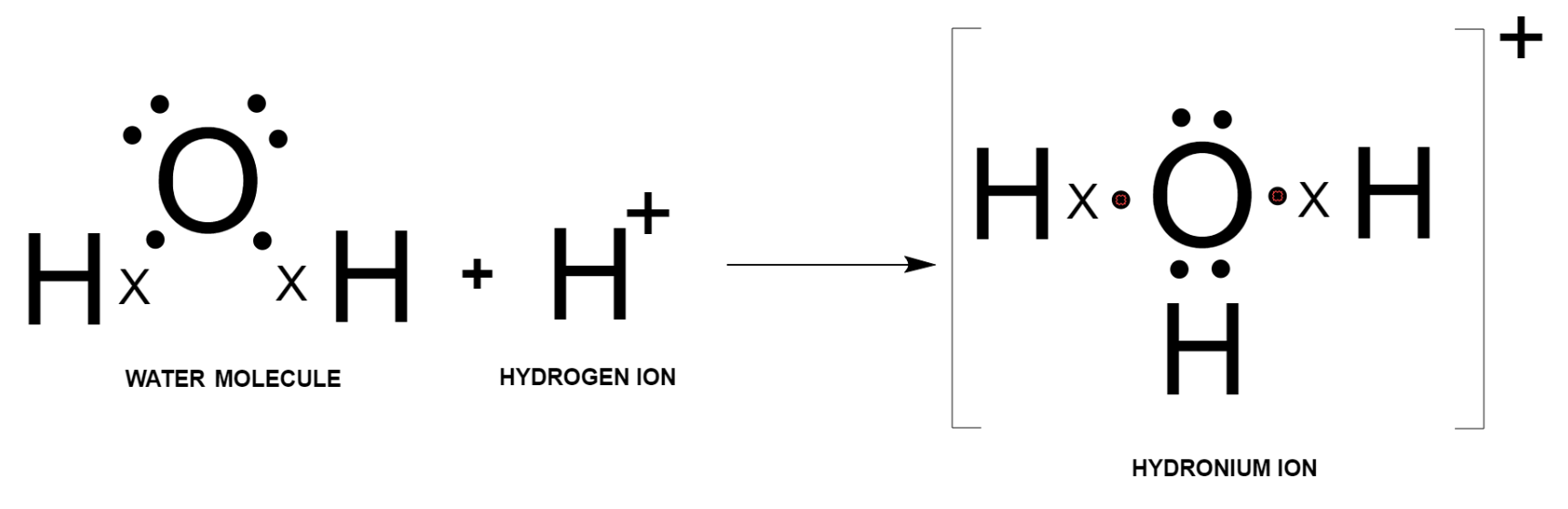
Hence, the electron dot diagram of hydronium ion is:
Note:
The octet rule is always followed during bond formation. According to this rule, every atom tends to attain 8 electrons in its outermost shell. But there is an exception in small atoms like hydrogen. Hydrogen can only attain up to 2 electrons in its valence shell.
Complete answer:
Every element has a specific number of electrons present in its atomic orbitals. Out of these electrons, only those participating in bond formation with another atom which is present in the outermost shell of the atom. These electrons which are present in the outermost orbital of an atom are called valence electrons.
Any atom forms a bond with another atom to attain stability by completing its octet. And it is not necessary that each electron present in the valence shell takes part in octet formation. In some cases, it has been observed that one or more pairs of electrons in the valence shell remain unshared during bond formation. Such pairs of electrons in the outermost shell of an atom that remains unshared are known as lone pairs of electrons.
Hydronium ion is made by the addition of an ${{\text{H}}^{+}}$ ion to a water molecule. To draw the electron dot or Lewis diagram of hydronium ion, we first need to write the electronic configuration.
\[\begin{align}
& \text{H}-1{{\text{s}}^{1}} \\
& \text{O}-1{{\text{s}}^{2}}2{{\text{s}}^{2}}2{{\text{p}}^{4}} \\
\end{align}\]
The oxygen has a total of 6 electrons in its valence shell out of which two electrons form a covalent bond with two hydrogen atoms and form a water molecule while the remaining two pairs of electrons exist as lone pairs of oxygen.

Now, this water molecule has two lone pairs of electrons. When an ${{\text{H}}^{+}}$ ion is added to a water molecule, one of the lone pairs of electrons is transferred from oxygen to hydrogen ion, and the formation of a coordinate bond takes place as shown below.

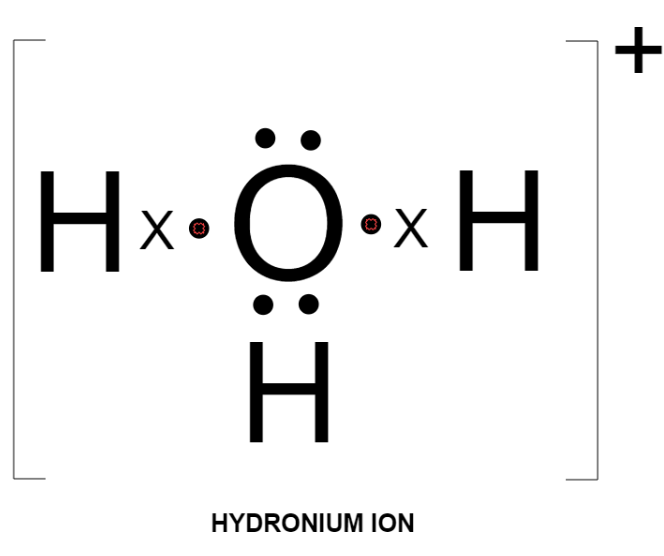
Hence, the electron dot diagram of hydronium ion is:

Note:
The octet rule is always followed during bond formation. According to this rule, every atom tends to attain 8 electrons in its outermost shell. But there is an exception in small atoms like hydrogen. Hydrogen can only attain up to 2 electrons in its valence shell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




