
When do we use the prefixes iso, neo, tert, sec when we name organic compounds?
Answer
478.5k+ views
Hint: the names of organic compounds follow the IUPAC nomenclature, but sometimes for ease, we follow common names of organic compounds which are different from IUPAC names. The common consist of some prefixes which indicates the spatial arrangement of functional groups (usually alkyl substitutions)
Complete answer:
The prefix "iso" is used when all carbons except one form a continuous chain. The prefix "neo" is used when all but two carbons form a continuous chain. The prefix "sec" or "s" is used when the functional; group is bonded to a secondary carbon (although this can be used only for the chain containing four or more carbons). The prefix "tert" or "t" is used when the functional group is bonded to a tertiary carbon.
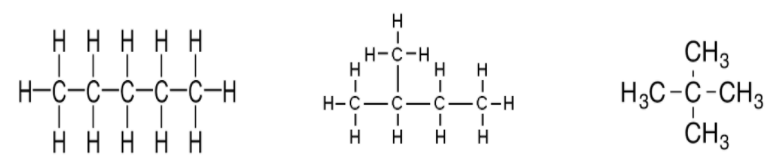
For example, the first structure is normal pentane, the second is isopentane and the third represents the neopentane, these follow the criteria for the prefixes.
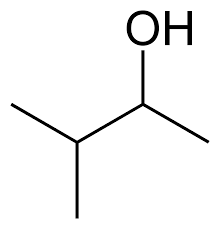
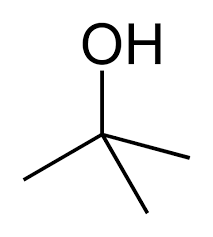
Now, the following structures represent the sec and tert butanol. The first structure is sec butanol and the second structure is tert butanol. In the first one, the functional group is attached to secondary carbon (carbon attached to two carbon atoms) and in the second structure, the functional group is attached to tertiary carbon atoms (carbon atoms attached to three carbon atoms).
Note:
The structure should be made carefully following the valency of carbon. Sometimes, these prefixes are also allowed to be used in the IUPAC names, for example,
5-(1-ethyl-1-isopropyl)nonane.
Complete answer:
The prefix "iso" is used when all carbons except one form a continuous chain. The prefix "neo" is used when all but two carbons form a continuous chain. The prefix "sec" or "s" is used when the functional; group is bonded to a secondary carbon (although this can be used only for the chain containing four or more carbons). The prefix "tert" or "t" is used when the functional group is bonded to a tertiary carbon.
For example, the first structure is normal pentane, the second is isopentane and the third represents the neopentane, these follow the criteria for the prefixes.

Now, the following structures represent the sec and tert butanol. The first structure is sec butanol and the second structure is tert butanol. In the first one, the functional group is attached to secondary carbon (carbon attached to two carbon atoms) and in the second structure, the functional group is attached to tertiary carbon atoms (carbon atoms attached to three carbon atoms).


Note:
The structure should be made carefully following the valency of carbon. Sometimes, these prefixes are also allowed to be used in the IUPAC names, for example,
5-(1-ethyl-1-isopropyl)nonane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




