
Using VBT, explain the type of hybridization, geometry and magnetic property of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$.
Answer
575.7k+ views
Hint: Generally valence bond theory (VBT) explains the overlapping of atomic orbitals involved in the formation of the molecule. According to valence bond theory bonds are nothing but weakly coupled orbitals.
Complete step by step answer:
- In the question it is given that to write the hybridization, geometry and magnetic property of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ .
- The IUPAC name of the compound is tetrachloronickelate (II).
- The central metal atom in the given complex is nickel.
- The atomic number of nickel is 28.
- The electronic configuration of nickel is as follows.
\[[Ar]3{{d}^{8}}4{{s}^{2}}\]
- The overlapping of atomic orbitals with chlorine atoms is as follows.
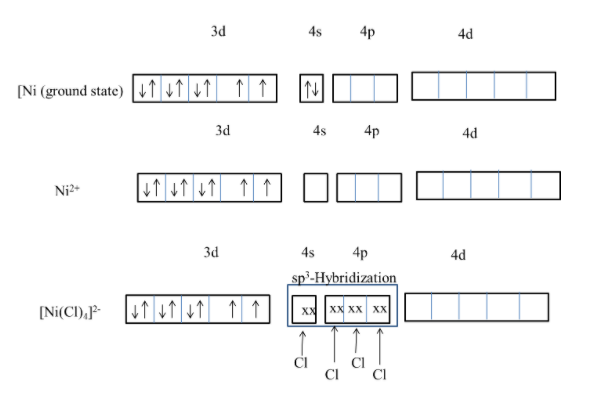
- From the above image we can say that the hybridization of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ is $s{{p}^{3}}$ .
- The four chlorine atoms donated the electrons to the 4s and 4p orbital of the nickel. This is called overlapping of ligands with the central metal orbitals.
- The geometry of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ is tetrahedral due to the weak ligand chlorine.
- ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ complex is paramagnetic in nature due to the presence of lone pair of electrons in 3d orbital of the central metal atom nickel.
Note: If the complex has unpaired electrons then it is called paramagnetic and if the complex has no unpaired electrons in the orbitals then the complex is called diamagnetic. Weak field ligands are going to form outer orbital complexes with the central metal atom and strong field ligands are going to form inner orbital complexes with central metal atoms.
Complete step by step answer:
- In the question it is given that to write the hybridization, geometry and magnetic property of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ .
- The IUPAC name of the compound is tetrachloronickelate (II).
- The central metal atom in the given complex is nickel.
- The atomic number of nickel is 28.
- The electronic configuration of nickel is as follows.
\[[Ar]3{{d}^{8}}4{{s}^{2}}\]
- The overlapping of atomic orbitals with chlorine atoms is as follows.

- From the above image we can say that the hybridization of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ is $s{{p}^{3}}$ .
- The four chlorine atoms donated the electrons to the 4s and 4p orbital of the nickel. This is called overlapping of ligands with the central metal orbitals.
- The geometry of ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ is tetrahedral due to the weak ligand chlorine.
- ${{\left[ NiC{{l}_{4}} \right]}^{2-}}$ complex is paramagnetic in nature due to the presence of lone pair of electrons in 3d orbital of the central metal atom nickel.
Note: If the complex has unpaired electrons then it is called paramagnetic and if the complex has no unpaired electrons in the orbitals then the complex is called diamagnetic. Weak field ligands are going to form outer orbital complexes with the central metal atom and strong field ligands are going to form inner orbital complexes with central metal atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




