
How many valence electrons are in an atom of Sulphur?
Answer
565.2k+ views
Hint The atomic number of Sulphur is 16 and it possesses 16 electrons in the atom. The valence electrons are the electrons that go into the outermost shell of an atom.
Complete answer:
In the question it is asked how many valence electrons are present in the Sulphur (S) atom. From the time we are learning about the periodic table we know the fact that the elements are placed in a periodic table in accordance with the increasing order of the atomic number. As the atomic number is increasing the electrons in the atoms are also increasing.
We know that the valence electrons are those electrons which enter into the last or the outermost shell of the atom and these are the electrons which actively take part in the chemical reaction by sharing or losing its electrons.
The atoms have the tendency to obtain the stable octet configuration or noble gas configuration either by gaining or losing the electrons and all these phenomena are restricted to the valence shell of the electron.
Now let’s discuss the number of electrons present in the S atom, its electronic configuration and about the valence electrons in them.
We know that the atomic number of S is 16 so there will be 16 electrons in the atom. Let’s write the electronic configuration of the S atom by distributing the electrons in various orbitals present in the shell in the increasing order of energy.
Electronic configuration of S is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$
So from the electronic configuration we know that the outermost shell in S atom is the third shell and the number of electrons present in the third shell accounts for the valence electrons present in S atom.
So there are two orbitals in the third shell which have electrons in them. In the s orbital there are 2 electrons and in the p orbital there are 4 electrons. Therefore there are in total 6 electrons in the valence shell.
Hence there are six valence electrons in the S atom.
The distribution of electrons in each shell of S atom is represented in the figure given below:
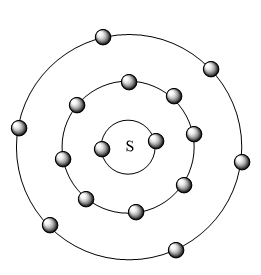
Note: The valency of S is -2 it is because the S atom needs only two more electrons to complete its octet configuration hence it gains two more electrons and completes its shell and will have a noble gas configuration. Hence it has a valency of -2.We can easily predict the valence electrons present in an atom if we know that the element belongs to which main group i.e. the S belongs to group 16 i.e. the main group ,group VI. Hence the elements in these groups have six valence electrons.
Complete answer:
In the question it is asked how many valence electrons are present in the Sulphur (S) atom. From the time we are learning about the periodic table we know the fact that the elements are placed in a periodic table in accordance with the increasing order of the atomic number. As the atomic number is increasing the electrons in the atoms are also increasing.
We know that the valence electrons are those electrons which enter into the last or the outermost shell of the atom and these are the electrons which actively take part in the chemical reaction by sharing or losing its electrons.
The atoms have the tendency to obtain the stable octet configuration or noble gas configuration either by gaining or losing the electrons and all these phenomena are restricted to the valence shell of the electron.
Now let’s discuss the number of electrons present in the S atom, its electronic configuration and about the valence electrons in them.
We know that the atomic number of S is 16 so there will be 16 electrons in the atom. Let’s write the electronic configuration of the S atom by distributing the electrons in various orbitals present in the shell in the increasing order of energy.
Electronic configuration of S is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}$
So from the electronic configuration we know that the outermost shell in S atom is the third shell and the number of electrons present in the third shell accounts for the valence electrons present in S atom.
So there are two orbitals in the third shell which have electrons in them. In the s orbital there are 2 electrons and in the p orbital there are 4 electrons. Therefore there are in total 6 electrons in the valence shell.
Hence there are six valence electrons in the S atom.
The distribution of electrons in each shell of S atom is represented in the figure given below:

Note: The valency of S is -2 it is because the S atom needs only two more electrons to complete its octet configuration hence it gains two more electrons and completes its shell and will have a noble gas configuration. Hence it has a valency of -2.We can easily predict the valence electrons present in an atom if we know that the element belongs to which main group i.e. the S belongs to group 16 i.e. the main group ,group VI. Hence the elements in these groups have six valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




