
What is the valency of silicon atom-
A. 4
B. 3
C. 2
D. 1
Answer
564.6k+ views
Hint: Atom’s chemical properties depend on the atomic number. The valency of an element depends upon the total number of electrons in the outermost shell of an atom. Valence electrons are the electrons that are present in the outermost shell of an atom.
Complete step by step answer:
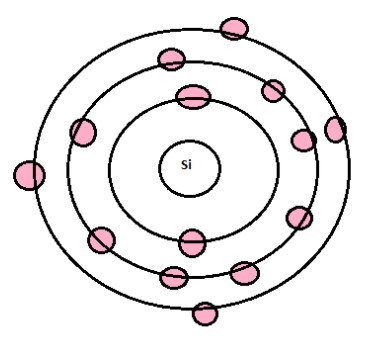
Silicon is a chemical element found in the periodic table which has the symbol Si with an atomic number 14. The atomic structure of silicon makes it an extremely important semiconductor. The atoms in silicon are held in fixed positions by strong forces and only have limited movement.
The valency is determined by the number of valence electrons present in the atom of that element.
Silicon has 4 electrons in the outermost shell. Hence, the valency of silicon atoms is 4.
Thus, the correct answer is option A. i.e., 4.
Additional Information:
Electronic configuration of Silicon is 2, 8, 4. Thus to complete its octet it either needs to lose 4 electrons or gain 4 electrons. Normally it does not do it because this requires a high amount of energy. But it does have the ability to show +4 or -4 valency. To complete the octet, it can share its 4 electrons, hence its valency is 4.
Note:
Silicon is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. In the periodic table, it is a member of group fourteen. Each silicon atom consists of two oxygen atoms. Carbon is present above the silicon atom and germanium; tin and lead are present below it.
Complete step by step answer:
Silicon is a chemical element found in the periodic table which has the symbol Si with an atomic number 14. The atomic structure of silicon makes it an extremely important semiconductor. The atoms in silicon are held in fixed positions by strong forces and only have limited movement.
The valency is determined by the number of valence electrons present in the atom of that element.
Silicon has 4 electrons in the outermost shell. Hence, the valency of silicon atoms is 4.

Thus, the correct answer is option A. i.e., 4.
Additional Information:
Electronic configuration of Silicon is 2, 8, 4. Thus to complete its octet it either needs to lose 4 electrons or gain 4 electrons. Normally it does not do it because this requires a high amount of energy. But it does have the ability to show +4 or -4 valency. To complete the octet, it can share its 4 electrons, hence its valency is 4.
Note:
Silicon is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid and semiconductor. In the periodic table, it is a member of group fourteen. Each silicon atom consists of two oxygen atoms. Carbon is present above the silicon atom and germanium; tin and lead are present below it.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




