
Valency of Sulphur in sulphuric acid is:
(a) 2
(b) 4
(c) 6
(d) 8
Answer
573.6k+ views
Hint: Valency may be defined as the combining power of any element to lose or gain electrons during the formation of either molecules or the compounds or during a chemical reaction. In sulphuric acid, Sulphur forms the covalent bonds with its neighbouring atoms. Find its valency.
Complete Solution :
- First of all, we should know what valency is. By the term valency we mean the combining capacity of an atom to lose or gain electrons to acquire the stable nearest noble gas configuration or stable electronic configuration. In case of metals, it is equal to the number of electrons which are present in the outermost shell of their atoms, and for non -metals it is eight minus the number of electrons which are present in the outermost shell of the atom. For example:- oxygen has 6 electrons in its outermost shell. Since oxygen is a non-metal. So, its valency is: 8 - 6 = 2.
- Sulphur is a non-metal belonging to the 16th group of the period table and belongs to the oxygen family and is a p-block element. It consists of six electrons in its outermost valence shell.
In case of sulfuric acid, Sulphur atom present in it forms six covalent bonds with its neighbouring atoms i.e. with the oxygen atoms as:
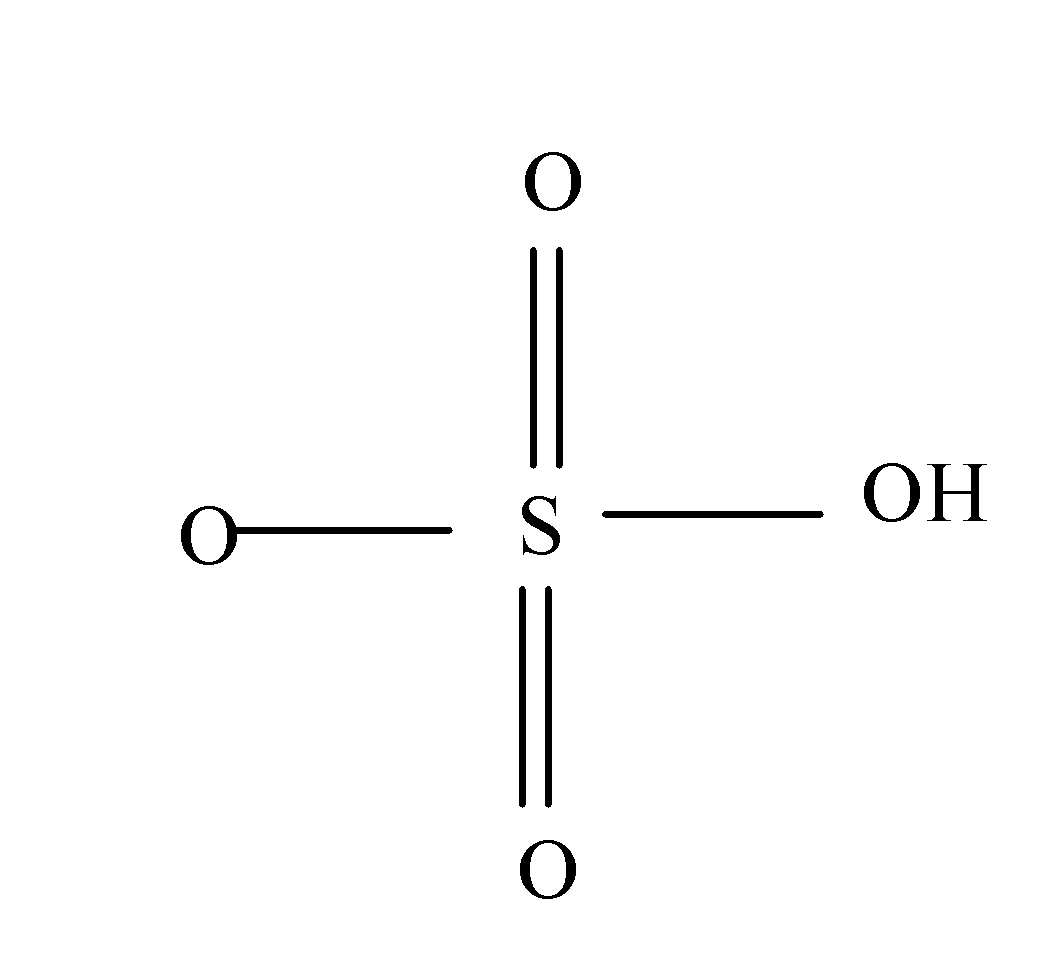
So, hence the valency of Sulphur in sulphuric acid is 6.
So, the correct answer is “Option C”.
Note: Don’t get confused in the term’s valency and the oxidation states. Valency is the number of electrons that are present in the outermost shell of an element . On the other hand , oxidation state is the number of electrons which an element gains or loses during the formation of the compound.
Complete Solution :
- First of all, we should know what valency is. By the term valency we mean the combining capacity of an atom to lose or gain electrons to acquire the stable nearest noble gas configuration or stable electronic configuration. In case of metals, it is equal to the number of electrons which are present in the outermost shell of their atoms, and for non -metals it is eight minus the number of electrons which are present in the outermost shell of the atom. For example:- oxygen has 6 electrons in its outermost shell. Since oxygen is a non-metal. So, its valency is: 8 - 6 = 2.
- Sulphur is a non-metal belonging to the 16th group of the period table and belongs to the oxygen family and is a p-block element. It consists of six electrons in its outermost valence shell.
In case of sulfuric acid, Sulphur atom present in it forms six covalent bonds with its neighbouring atoms i.e. with the oxygen atoms as:

So, hence the valency of Sulphur in sulphuric acid is 6.
So, the correct answer is “Option C”.
Note: Don’t get confused in the term’s valency and the oxidation states. Valency is the number of electrons that are present in the outermost shell of an element . On the other hand , oxidation state is the number of electrons which an element gains or loses during the formation of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




