
Why is Van der Waals radius greater than covalent radius for covalent compounds?
Answer
483.9k+ views
Hint: Vander wall radius can be measured when there is no bonding between two atoms while covalent radius is measured when two atoms are bonded with each other by a covalent bond. The bonding between atoms in covalent bond is stronger as compared to Vander wall.
Complete answer:
When two atoms come towards each other then they mate from a bond which depends on the electronic configuration and available valence electrons in each of them. Thus when two atoms of the same element do not form any bond with each other but they are close enough to each other then we measure the distance between their nucleus which is known as Vander waal radius.
Thus in the Vander waal radius there is no bonding between the atoms but they are close enough to each other. It can be shown as:
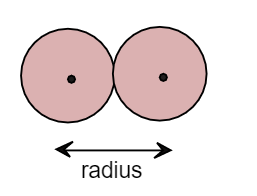
When two atoms come towards each other and form a covalent bond by overlapping the atomic orbital then the radius between the nucleus of atoms is called covalent radius. Thus while calculating covalent radius the atoms overlap each other and form a covalent with each other. It can be shown as:
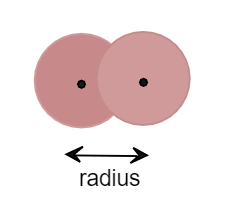
Thus, we can say that van der Waals radius is greater than covalent radius for covalent compounds.
Note:
It must be noted that while calculating the radius of a compound it must be calculated from the centre of the atom which is the nucleus of the atom to the nucleus of another atom. Vander wall radius cannot be less than covalent radius as covalent bond is formed by overlapping of atoms which will ultimately reduce its distance between the nucleus to another nucleus.
Complete answer:
When two atoms come towards each other then they mate from a bond which depends on the electronic configuration and available valence electrons in each of them. Thus when two atoms of the same element do not form any bond with each other but they are close enough to each other then we measure the distance between their nucleus which is known as Vander waal radius.
Thus in the Vander waal radius there is no bonding between the atoms but they are close enough to each other. It can be shown as:

When two atoms come towards each other and form a covalent bond by overlapping the atomic orbital then the radius between the nucleus of atoms is called covalent radius. Thus while calculating covalent radius the atoms overlap each other and form a covalent with each other. It can be shown as:

Thus, we can say that van der Waals radius is greater than covalent radius for covalent compounds.
Note:
It must be noted that while calculating the radius of a compound it must be calculated from the centre of the atom which is the nucleus of the atom to the nucleus of another atom. Vander wall radius cannot be less than covalent radius as covalent bond is formed by overlapping of atoms which will ultimately reduce its distance between the nucleus to another nucleus.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




