
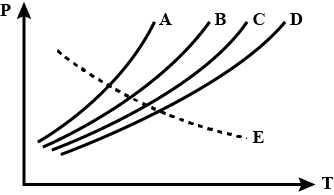
Vapor pressure diagram of some liquids plotted against temperature are shown below. Most volatile liquid is

Answer
532.2k+ views
Hint: To determine the most volatile liquid, we first need to know what volatile liquids are. The liquids which can easily convert to a vapor state at a certain temperature are known as volatile liquids. Liquids like perfumes, alcohols, acetones, etc., are examples of volatile liquids.
Complete answer:
The volatility of a liquid is dependent on the vapor pressure of a liquid. The higher the vapor pressure of the liquid, the more volatile it is at room temperature.
The pressure exerted by the evaporated liquids or vapors is known as vapor pressure. This pressure exerted is measured when the system is in thermodynamic equilibrium and is at a certain temperature.
The vapor pressure of a liquid depends on the nature of the liquid and the temperature of the system.
The weaker the intermolecular forces of a liquid are, the easier it is to convert them into vapor and hence increase the vapor pressure. This is why the vapor pressure of liquids like alcohols and acetones is higher than water.
Now, the given graph shows the dependence of vapor pressure on the temperature of different compounds.
Since the curve of compound A changes the most at the minimum change in temperature, we can say that with a small change in temperature, the liquid can be easily turned into vapors, and hence it is the most volatile liquid.
So, the answer is an option (A).
Additional information:
Raoult's law defines the partial pressure of an ideal mixture and can be given by
\[{{P}_{total}}={{P}_{1}}^{o}+({{P}_{2}}^{o}-{{P}_{1}}^{o}){{x}_{2}}\]
Where $P{}^\circ $ are the vapor pressures of the component in pure state and x is the mole fraction of the component.
Note:
It should be noted that with an increase in temperature, the kinetic energy of the molecules of liquid increases. The increase in kinetic energy increases the rate of transition of molecules in the liquid into vapor and hence the vapor pressure also increases.
Complete answer:
The volatility of a liquid is dependent on the vapor pressure of a liquid. The higher the vapor pressure of the liquid, the more volatile it is at room temperature.
The pressure exerted by the evaporated liquids or vapors is known as vapor pressure. This pressure exerted is measured when the system is in thermodynamic equilibrium and is at a certain temperature.
The vapor pressure of a liquid depends on the nature of the liquid and the temperature of the system.
The weaker the intermolecular forces of a liquid are, the easier it is to convert them into vapor and hence increase the vapor pressure. This is why the vapor pressure of liquids like alcohols and acetones is higher than water.
Now, the given graph shows the dependence of vapor pressure on the temperature of different compounds.
Since the curve of compound A changes the most at the minimum change in temperature, we can say that with a small change in temperature, the liquid can be easily turned into vapors, and hence it is the most volatile liquid.
So, the answer is an option (A).
Additional information:
Raoult's law defines the partial pressure of an ideal mixture and can be given by
\[{{P}_{total}}={{P}_{1}}^{o}+({{P}_{2}}^{o}-{{P}_{1}}^{o}){{x}_{2}}\]
Where $P{}^\circ $ are the vapor pressures of the component in pure state and x is the mole fraction of the component.
Note:
It should be noted that with an increase in temperature, the kinetic energy of the molecules of liquid increases. The increase in kinetic energy increases the rate of transition of molecules in the liquid into vapor and hence the vapor pressure also increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




