
Various products formed on oxidation of 2,5- dimethylhexan-3-one are :
(i)
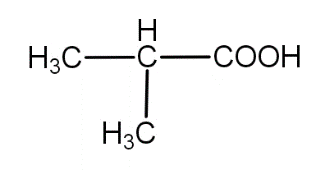
(ii)
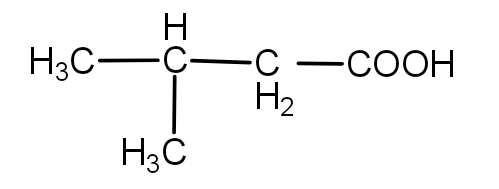
(iii) $C{H_3}COOH$
(iv) HCOOH
a.) (i) and (ii)
b.) (i), (ii) and (iii)
c.) (i), (ii), (iii) and (iv)
d.) (iii) and (iv)


Answer
560.7k+ views
Hint The oxidation of ketones involves carbon-carbon bond cleavage. The substrate may cleave into two different reagents that may get oxidized. Normally, the ketone is oxidised to give carboxylic acid. The various reagents in which the substrate has cleaved will also get oxidised on the ketone part resulting in a variety of carboxylic acids.
Complete step by step answer :
First, let us see what oxidation is. The oxidation is defined as the process of addition of oxygen or the removal of hydrogen from the substrate molecule. In terms of electrons, it is the loss of electrons from the substrate.
As we have studied that the oxidation of ketones involves carbon-carbon bond cleavage.
The reaction involved is mentioned below:
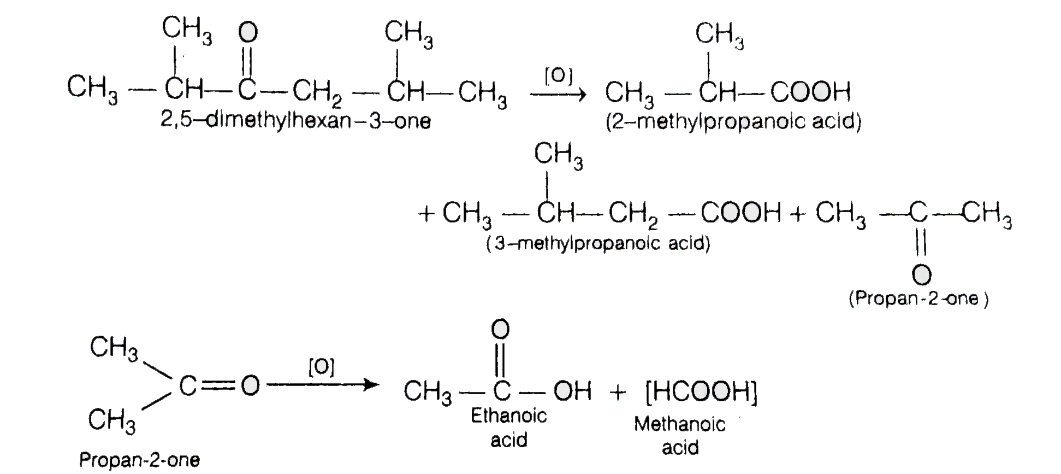
The substrate given to us i.e. 2,5- dimethylhexan-3-one is a ketone. So, its oxidation can also involve carbon-carbon bond cleavage. This oxidation may result in the formation of a number of products as - ( 2- Methylpropanoic acid), (3- Methylbutanoic acid), $C{H_3}COOH$
(Ethanoic acid) and HCOOH ( formic acid). The formic acid is formed from the propane.
So, the correct answer is the option c.
Note: It must be noted that oxidation occurs when the hydrogen is abstracted or the oxygen is added to the substrate. Whenever a substrate gets oxidised, there will be some other molecule that has got reduced because of oxidation and reduction, both processes move simultaneously.
Complete step by step answer :
First, let us see what oxidation is. The oxidation is defined as the process of addition of oxygen or the removal of hydrogen from the substrate molecule. In terms of electrons, it is the loss of electrons from the substrate.
As we have studied that the oxidation of ketones involves carbon-carbon bond cleavage.
The reaction involved is mentioned below:

The substrate given to us i.e. 2,5- dimethylhexan-3-one is a ketone. So, its oxidation can also involve carbon-carbon bond cleavage. This oxidation may result in the formation of a number of products as - ( 2- Methylpropanoic acid), (3- Methylbutanoic acid), $C{H_3}COOH$
(Ethanoic acid) and HCOOH ( formic acid). The formic acid is formed from the propane.
So, the correct answer is the option c.
Note: It must be noted that oxidation occurs when the hydrogen is abstracted or the oxygen is added to the substrate. Whenever a substrate gets oxidised, there will be some other molecule that has got reduced because of oxidation and reduction, both processes move simultaneously.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




