
What is the VSEPR shape of the molecule $ P{{F}_{3}} $ ?
Answer
531k+ views
Hint : In order to solve the given problem and find the shape of the molecule; we will use the renowned VSEPR’s theory. We will firstly study in brief about the VSEPR’s theory along with its rules for different types of structures. Further we will study the particular case of three bond pairs and one lone pair with the help of an example relevant to this type.
Complete Step By Step Answer:
The model used in chemistry to determine the geometry of individual molecules from the number of electron pairs circling their core atoms is the Valence shell electron pair repulsion theory, or VSEPR theory. The Gillespie-Nyholm theory is also named after its two principal creators, Ronald Gillespie and Ronald Nyholm. It is based on the fact that there is a repulsion of both atoms between the pairs of valence electrons, and the atoms will therefore appear to organize themselves in a manner that minimizes the repulsion of this electron pair. The geometry of the resulting molecule is determined by this arrangement of the atom.
There are a set of rules and postulates for different structures according to this theory. This theory also predicts the shape for different sample structures. VSEPR stands for valence shell electron pair repulsion. This theory basically says that bonding and nonbonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional spaces and this gives the molecules their shape.
We can use the following notations when examining a Lewis structure of a molecule.
$ A\text{ }= $ Central atom
$ X\text{ }= $ peripheral atoms
$ E\text{ }= $ non-bonding electron pairs of the central atom
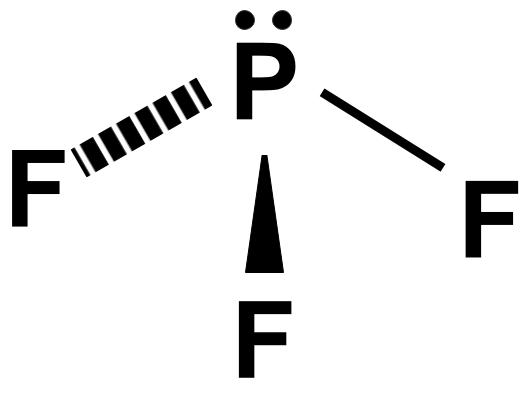
We can use VSEPR theory to predict a trigonal pyramidal shape for the molecule $ P{{F}_{3}} $ because of its $ A{{X}_{3}}E $ status.
Note :
Remember that the number of electron pairs is the number of lone pairs and the number of bond pairs determines the shape of the given molecule. According to this VSEPR’s theory the molecule having three bond pairs and one lone pair will have a triangular pyramidal structure where the three bonds are present as the base of the pyramid and lone pair at the top.
Complete Step By Step Answer:
The model used in chemistry to determine the geometry of individual molecules from the number of electron pairs circling their core atoms is the Valence shell electron pair repulsion theory, or VSEPR theory. The Gillespie-Nyholm theory is also named after its two principal creators, Ronald Gillespie and Ronald Nyholm. It is based on the fact that there is a repulsion of both atoms between the pairs of valence electrons, and the atoms will therefore appear to organize themselves in a manner that minimizes the repulsion of this electron pair. The geometry of the resulting molecule is determined by this arrangement of the atom.
There are a set of rules and postulates for different structures according to this theory. This theory also predicts the shape for different sample structures. VSEPR stands for valence shell electron pair repulsion. This theory basically says that bonding and nonbonding electron pairs of the central atom in a molecule will repel (push away from) each other in three dimensional spaces and this gives the molecules their shape.
We can use the following notations when examining a Lewis structure of a molecule.
$ A\text{ }= $ Central atom
$ X\text{ }= $ peripheral atoms
$ E\text{ }= $ non-bonding electron pairs of the central atom
We can use VSEPR theory to predict a trigonal pyramidal shape for the molecule $ P{{F}_{3}} $ because of its $ A{{X}_{3}}E $ status.

Note :
Remember that the number of electron pairs is the number of lone pairs and the number of bond pairs determines the shape of the given molecule. According to this VSEPR’s theory the molecule having three bond pairs and one lone pair will have a triangular pyramidal structure where the three bonds are present as the base of the pyramid and lone pair at the top.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




