
Water and benzene have the same surface tension.
A.True
B.False
Answer
575.7k+ views
Hint: We know that surface tension is the phenomenon in which liquid particles at the surface are attracted by the bulk of the liquid that cause the reduction in surface area. The unit used to measure surface tension is dynes/cm.
Complete step by step answer:
Let’s first understand the structure of benzene and water molecules. Benzene is a compound of molecular formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}$ and structure of benzene is,
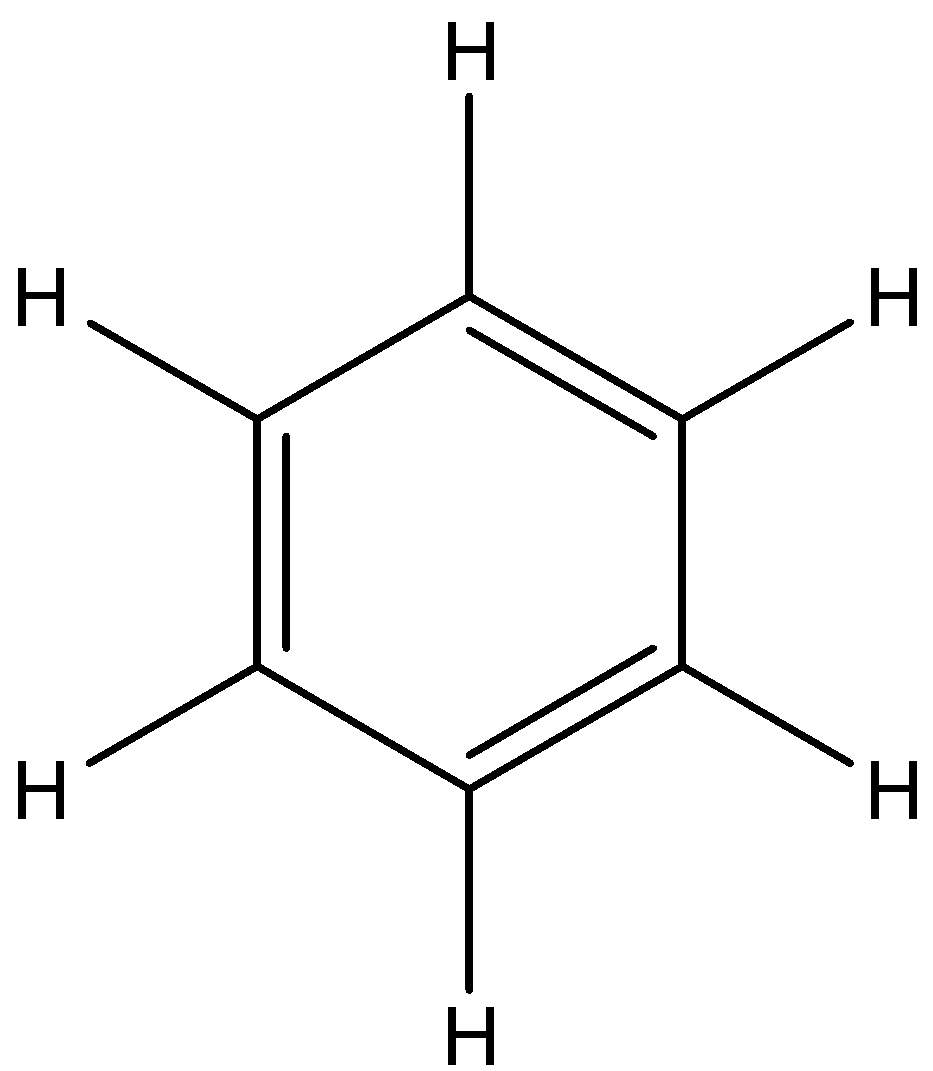
Water is a compound of molecular formula ${{\rm{H}}_{\rm{2}}}{\rm{O}}$. The structure of water is,
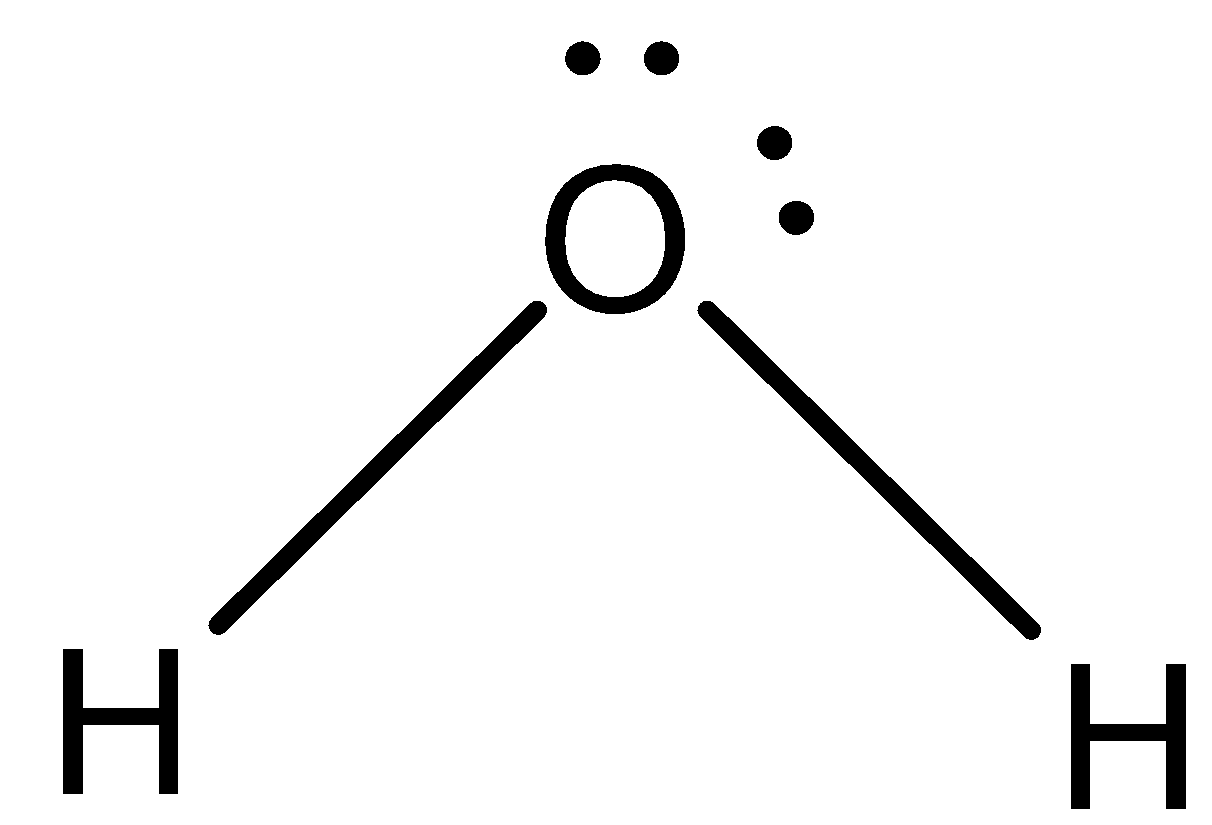
Now, come to the question. The surface tension is because of the intermolecular force within a substance. So, more intermolecular force of attraction means more surface tension of the compound.
Now, we have to compare the surface tension of benzene and water. Benzene is a nonpolar molecule as the bonding in benzene is C-H and C-C and there is not much difference of electronegativity between carbon and hydrogen atoms. In the benzene, there is also no hydrogen bonding and dipole. So, the intermolecular force of attraction in benzene is the weakest force that is London's dispersion force. So, the surface tension in benzene is low.
Water is a polar molecule. It also has four intermolecular hydrogen bonding. The hydrogen bonding in water can be shown as below:
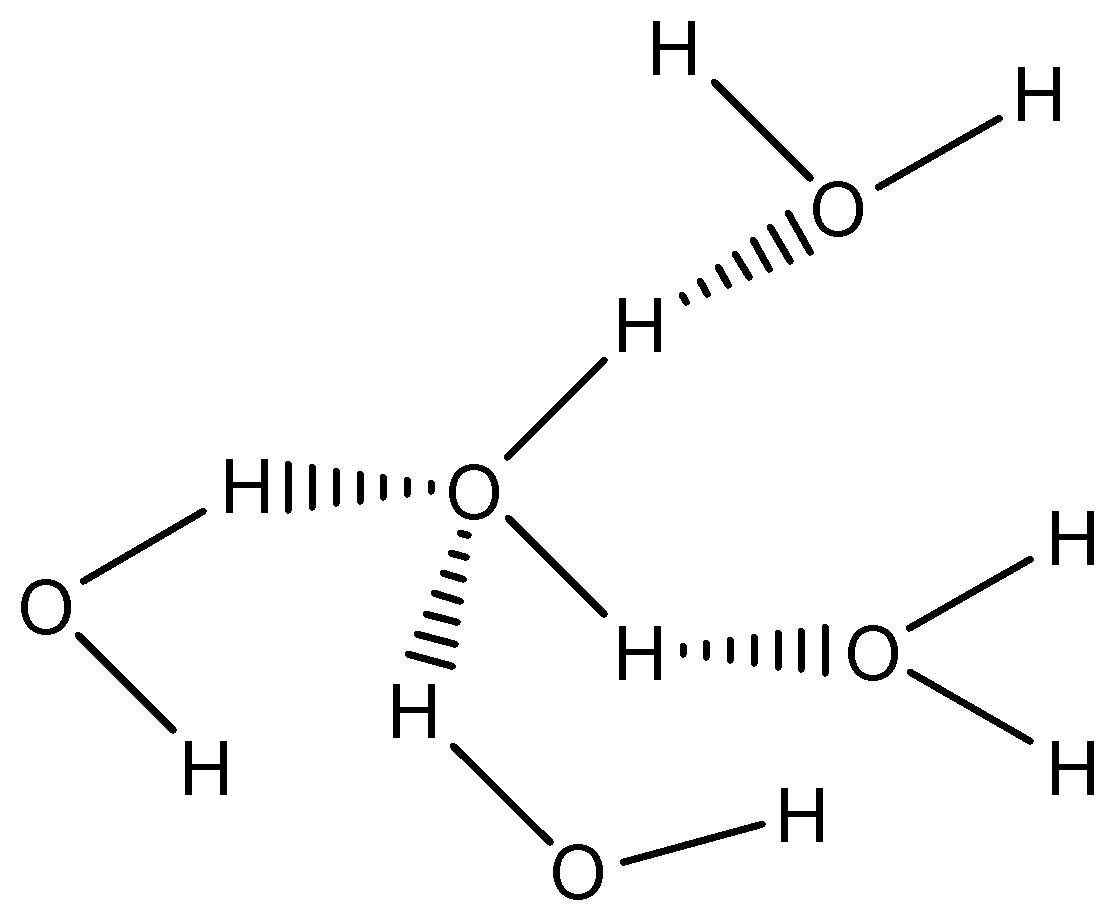
Due to the four intermolecular hydrogen bonding, intermolecular force in water is very high. So, the surface tension in water is very high.
Therefore, water and benzene have different surface tension.
Hence, correct answer is option B, that is, false.
Note: Hydrogen bond is a chemical bond in which formation of a covalent link of hydrogen atoms with other electronegative atoms, such as, fluorine, nitrogen and oxygen atoms takes place in the same or another molecule.
Complete step by step answer:
Let’s first understand the structure of benzene and water molecules. Benzene is a compound of molecular formula ${{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}$ and structure of benzene is,

Water is a compound of molecular formula ${{\rm{H}}_{\rm{2}}}{\rm{O}}$. The structure of water is,

Now, come to the question. The surface tension is because of the intermolecular force within a substance. So, more intermolecular force of attraction means more surface tension of the compound.
Now, we have to compare the surface tension of benzene and water. Benzene is a nonpolar molecule as the bonding in benzene is C-H and C-C and there is not much difference of electronegativity between carbon and hydrogen atoms. In the benzene, there is also no hydrogen bonding and dipole. So, the intermolecular force of attraction in benzene is the weakest force that is London's dispersion force. So, the surface tension in benzene is low.
Water is a polar molecule. It also has four intermolecular hydrogen bonding. The hydrogen bonding in water can be shown as below:

Due to the four intermolecular hydrogen bonding, intermolecular force in water is very high. So, the surface tension in water is very high.
Therefore, water and benzene have different surface tension.
Hence, correct answer is option B, that is, false.
Note: Hydrogen bond is a chemical bond in which formation of a covalent link of hydrogen atoms with other electronegative atoms, such as, fluorine, nitrogen and oxygen atoms takes place in the same or another molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




