
water exists in ____________ states of matter.
Answer
578.4k+ views
Hint: Water is a pure, odourless, colourless liquid. It is a life. Water freezes at the $\text{ }{{\text{0}}^{\text{0}}}\text{C }$. At room temperature, the water exists as a free-flowing substance. On heating at the $\text{ 10}{{\text{0}}^{\text{0}}}\text{C }$ temperature, the water molecules acquire the kinetic energy and converts into steam.
Complete step by step answer:
Water is life. It is an inorganic, transparent, tasteless, and odourless substance. It has a chemical formula as $\text{ }{{\text{H}}_{\text{2}}}\text{O }$. It is a polar compound. The general molecular structure of the water is as shown below,
The orientation of hydrogen bonds in the water is responsible for the various states of the matter. The three states of water are given as follows:
1) Gaseous state: When water is boiled, the water molecules acquire kinetic energy. The kinetic motion of the molecules causes the hydrogen bond to break. This allows the water molecule to escape to the air as the gas. This gaseous form is also known as water vapour. The water in the liquid is converted into a water vapour or steam when the water is boiled at the $\text{ 10}{{\text{0}}^{\text{0}}}\text{C }$ temperature.
2) Liquid state: The liquid state of water is very abundant on the earth. $\text{ 71}{\scriptstyle{}^{0}/{}_{0}}\text{ }$ Of the earth's surface is covered with liquid water. It is available as seawater, river or lakes. At room temperature, water exists as a liquid state. In water, the liquid is made of the tiny vibrating molecules of water which are held together by intermolecular hydrogen bonding. The hydrogen bonding between the water molecules is given as,
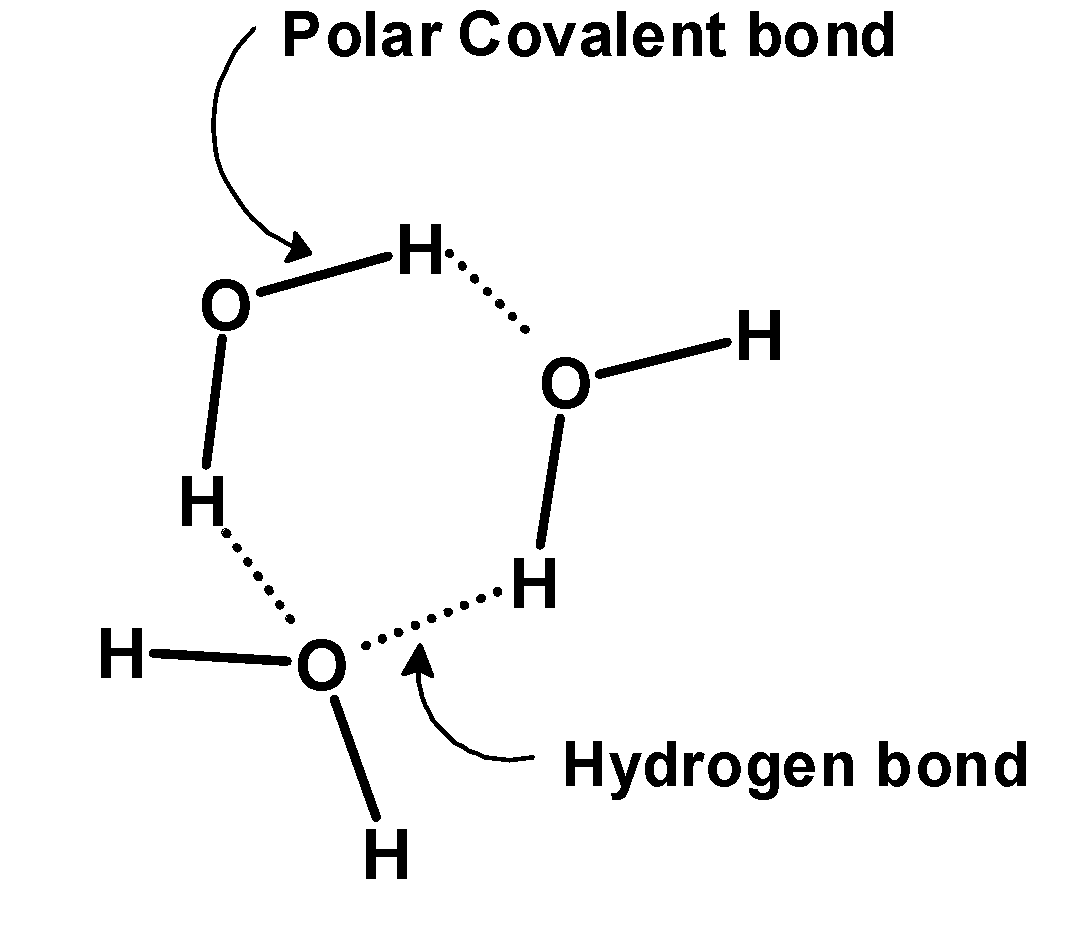
The hydrogen bonding holds the molecule together and the water exists in the liquid state. It is a free-flowing state of water. The liquid water is termed as the ‘life ‘. It is essential for the good upbringing of organisms.
3) Solid-state: When liquid state water is cooling down below the temperature $\text{ }{{\text{0}}^{\text{0}}}\text{C }$ , the water starts to freeze. This converts the liquid-solid into the solid forms. Thus ice is the solid-state of water. On a freezing, the water molecules exist in the crystalline structure. The water structure has empty spaces that are filled with the air .Therefore on freezing, the solidified water has the air in it, making it less dense than the water. Thus, ice floats on the liquid water surface. Three states of matter are interconvertible into each other. This is as shown below,
$\text{ }\begin{matrix}
\text{Ice} & \underset{\text{Freezing}}{\overset{\text{Melting}}{\longleftrightarrow}} & \text{Water} & \underset{\text{Condensation}}{\overset{\text{Vaporisation}}{\longleftrightarrow}} & \text{Water steam} \\
\text{(Solid)} & {} & \text{(Liquid)} & {} & \text{(Gas)} \\
\end{matrix}$
Thus, water exists in three states of matter.
Note: Note that, recent discoveries have confirmed that matter has two more states of matter .these are plasma and bose-einstein.in plasma state is a unique state of matter in which atoms are ionized and forms free moving positive and negative charge.it is difficult to convert the water into the plasma state. As water is a neutral molecule.it does not exist as ions in the vapour form.
Complete step by step answer:
Water is life. It is an inorganic, transparent, tasteless, and odourless substance. It has a chemical formula as $\text{ }{{\text{H}}_{\text{2}}}\text{O }$. It is a polar compound. The general molecular structure of the water is as shown below,

The orientation of hydrogen bonds in the water is responsible for the various states of the matter. The three states of water are given as follows:
1) Gaseous state: When water is boiled, the water molecules acquire kinetic energy. The kinetic motion of the molecules causes the hydrogen bond to break. This allows the water molecule to escape to the air as the gas. This gaseous form is also known as water vapour. The water in the liquid is converted into a water vapour or steam when the water is boiled at the $\text{ 10}{{\text{0}}^{\text{0}}}\text{C }$ temperature.
2) Liquid state: The liquid state of water is very abundant on the earth. $\text{ 71}{\scriptstyle{}^{0}/{}_{0}}\text{ }$ Of the earth's surface is covered with liquid water. It is available as seawater, river or lakes. At room temperature, water exists as a liquid state. In water, the liquid is made of the tiny vibrating molecules of water which are held together by intermolecular hydrogen bonding. The hydrogen bonding between the water molecules is given as,

The hydrogen bonding holds the molecule together and the water exists in the liquid state. It is a free-flowing state of water. The liquid water is termed as the ‘life ‘. It is essential for the good upbringing of organisms.
3) Solid-state: When liquid state water is cooling down below the temperature $\text{ }{{\text{0}}^{\text{0}}}\text{C }$ , the water starts to freeze. This converts the liquid-solid into the solid forms. Thus ice is the solid-state of water. On a freezing, the water molecules exist in the crystalline structure. The water structure has empty spaces that are filled with the air .Therefore on freezing, the solidified water has the air in it, making it less dense than the water. Thus, ice floats on the liquid water surface. Three states of matter are interconvertible into each other. This is as shown below,
$\text{ }\begin{matrix}
\text{Ice} & \underset{\text{Freezing}}{\overset{\text{Melting}}{\longleftrightarrow}} & \text{Water} & \underset{\text{Condensation}}{\overset{\text{Vaporisation}}{\longleftrightarrow}} & \text{Water steam} \\
\text{(Solid)} & {} & \text{(Liquid)} & {} & \text{(Gas)} \\
\end{matrix}$
Thus, water exists in three states of matter.
Note: Note that, recent discoveries have confirmed that matter has two more states of matter .these are plasma and bose-einstein.in plasma state is a unique state of matter in which atoms are ionized and forms free moving positive and negative charge.it is difficult to convert the water into the plasma state. As water is a neutral molecule.it does not exist as ions in the vapour form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




