
How do water molecules act like "little magnets"?
Answer
564.9k+ views
Hint: To answer this question we have to understand the structure and the polar nature of water molecules. Water is used as solvent for most of the reactions and it also plays a vital role in living organisms.
Complete answer:
Water is a simple molecule which consists of one oxygen atom bonded to two hydrogen atoms. The chemical formula of water is ${{H}_{2}}O$. Firstly, the structure of water was predicted to be linear but later with help of VSEPR theory it was found that the structure of water is bent. The structure of ${{H}_{2}}O$ is bent because of the presence of two lone pairs on oxygen.
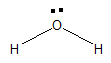
The oxygen is more electronegative than hydrogen atom. So, the oxygen atom possesses a partial negative charge and the hydrogen atom possesses positive negative charge. Therefore, water is polar in nature.
Since it is polar in nature the oxygen atom which possesses partial negative ($-\delta $) can attract protons which possess positive charge or any element or atom that possess positive charge on it.
Same goes with hydrogen, which possesses partial positive charge ($+\delta $) and can attract oxygen in other water molecules or any other atom that possesses a negative atom.
Basically, when a magnet is brought nearer to another magnet it attracts each other. Unlike charges, they attract each other. This is the same that happens in case of Water molecules too.
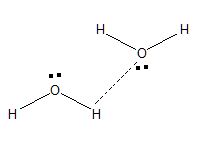
This is the reason why the water molecule is said to act like a little magnet.
Note:
The polarity of water molecules the point that accounts for its mechanism as a little magnet. Since water molecules can form clusters as illustrated above they are used as solvent for many reactions. If the structure of the water molecule is linear the dipole moment in the water molecule will be 0. Since the water molecule has a bent structure it possesses some dipole moment.
Complete answer:
Water is a simple molecule which consists of one oxygen atom bonded to two hydrogen atoms. The chemical formula of water is ${{H}_{2}}O$. Firstly, the structure of water was predicted to be linear but later with help of VSEPR theory it was found that the structure of water is bent. The structure of ${{H}_{2}}O$ is bent because of the presence of two lone pairs on oxygen.

The oxygen is more electronegative than hydrogen atom. So, the oxygen atom possesses a partial negative charge and the hydrogen atom possesses positive negative charge. Therefore, water is polar in nature.
Since it is polar in nature the oxygen atom which possesses partial negative ($-\delta $) can attract protons which possess positive charge or any element or atom that possess positive charge on it.
Same goes with hydrogen, which possesses partial positive charge ($+\delta $) and can attract oxygen in other water molecules or any other atom that possesses a negative atom.
Basically, when a magnet is brought nearer to another magnet it attracts each other. Unlike charges, they attract each other. This is the same that happens in case of Water molecules too.

This is the reason why the water molecule is said to act like a little magnet.
Note:
The polarity of water molecules the point that accounts for its mechanism as a little magnet. Since water molecules can form clusters as illustrated above they are used as solvent for many reactions. If the structure of the water molecule is linear the dipole moment in the water molecule will be 0. Since the water molecule has a bent structure it possesses some dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




