
We can distinguish by bromine test is:
(A) Alkenes
(B) Alkynes
(C) Alkanes
(D) Both A and B
Answer
585.9k+ views
Hint: The reaction is a type of addition reaction. So one type of hydrocarbon does not undergo addition reaction. Bromine water is a coloured solution which on reaction with a particular type of hydrocarbons will get decolourized.
Complete step by step solution:
Bromine test: This test is used to detect unsaturation in compounds. This is a type of addition reaction. For this reaction, there is a need for bromine water.
Bromine water is also called bromate solution or bromine solution. It has a chemical formula of ${\text{B}}{{\text{r}}_{\text{2}}}$. The only non-metal which is in the liquid state is bromine. It is yellowish in colour. Bromine is said to have very good oxidizing property.
Bromine water can be prepared in a laboratory by mixing fumes of Bromine with water. This method is not safe. Bromine water can also be prepared by ionization of ${\text{NaBr}}$ in presence of bleach and hydrochloric acid. The bromine water test is used for detection of unsaturation in the compounds. For example, when an alkene is reacted with bromine water, the bromine molecule undergoes addition reaction. Bromine is added across the double bond of the alkene. Experimentally bromine water which is yellowish in colour, after adding it undergoes decolourization. The colour change that takes place is from yellow to colourless.
The reaction is as follows:
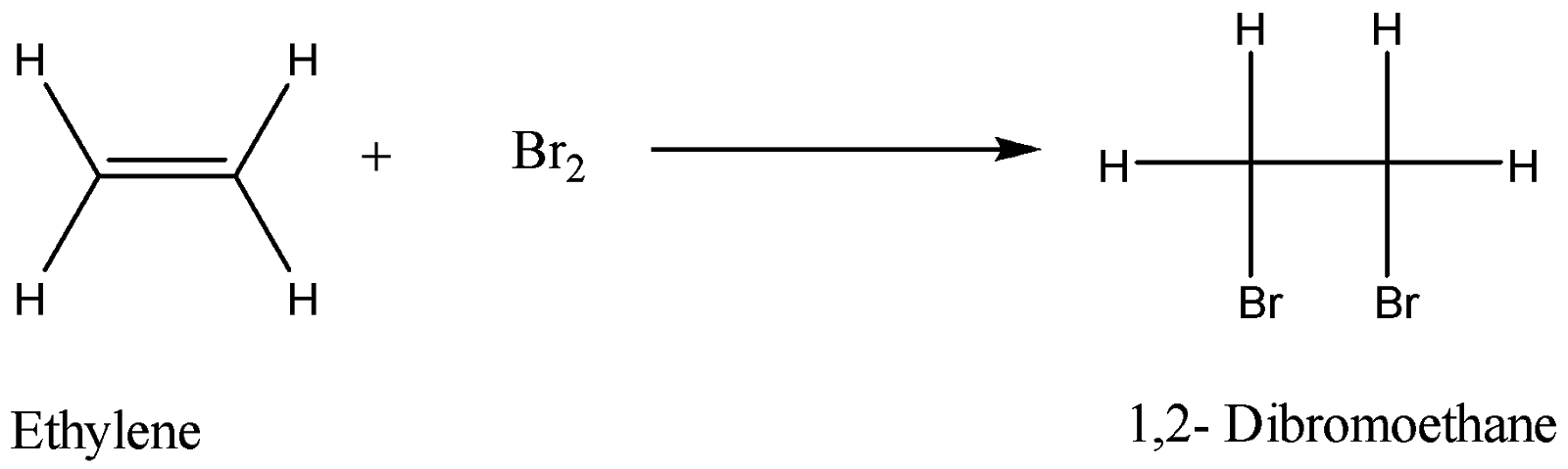
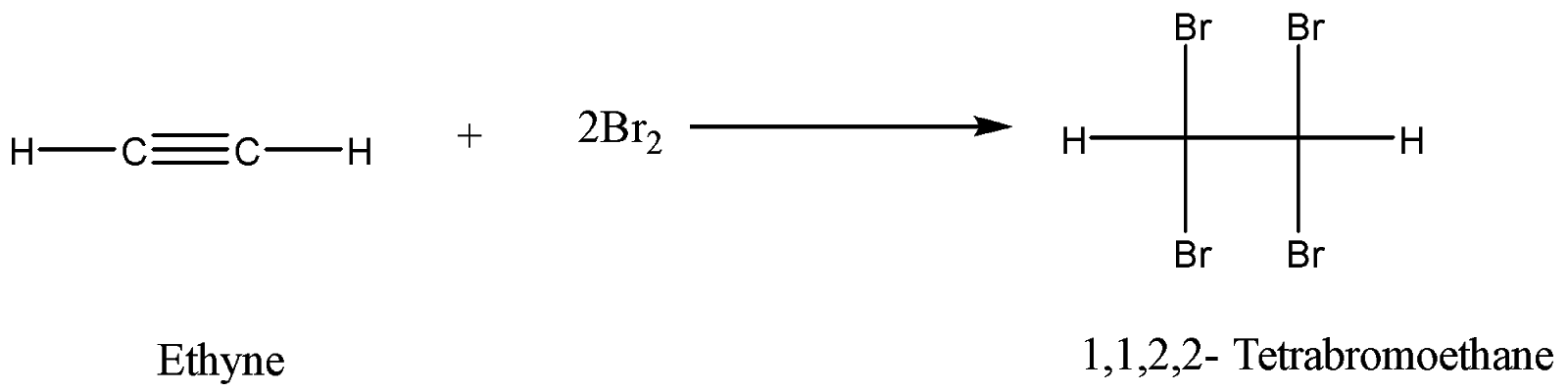
Alkynes also decolourize bromine water. Here alkynes will require two moles of bromine. One mole of bromine will convert alkyne to alkene and then the addition of another mole of bromine will completely saturate the compound. The reaction is as follows:
Alkane on the other hand does not react with bromine water. There is no decolourisation in this case. This is because alkanes are saturated compounds where they cannot undergo addition reactions. Bromine water is also used to check the presence of aldehyde groups in the compounds. In this reaction also there is decolourisation of bromine water.
So the correct option is (D).
Note: Bromine water test works by halogenation mechanism. Bromine water test takes place at room temperature in case of gaseous alkenes but in case of liquid alkenes, the reaction will take place in presence of \[{\text{CC}}{{\text{l}}_{\text{4}}}\].
Complete step by step solution:
Bromine test: This test is used to detect unsaturation in compounds. This is a type of addition reaction. For this reaction, there is a need for bromine water.
Bromine water is also called bromate solution or bromine solution. It has a chemical formula of ${\text{B}}{{\text{r}}_{\text{2}}}$. The only non-metal which is in the liquid state is bromine. It is yellowish in colour. Bromine is said to have very good oxidizing property.
Bromine water can be prepared in a laboratory by mixing fumes of Bromine with water. This method is not safe. Bromine water can also be prepared by ionization of ${\text{NaBr}}$ in presence of bleach and hydrochloric acid. The bromine water test is used for detection of unsaturation in the compounds. For example, when an alkene is reacted with bromine water, the bromine molecule undergoes addition reaction. Bromine is added across the double bond of the alkene. Experimentally bromine water which is yellowish in colour, after adding it undergoes decolourization. The colour change that takes place is from yellow to colourless.
The reaction is as follows:

Alkynes also decolourize bromine water. Here alkynes will require two moles of bromine. One mole of bromine will convert alkyne to alkene and then the addition of another mole of bromine will completely saturate the compound. The reaction is as follows:

Alkane on the other hand does not react with bromine water. There is no decolourisation in this case. This is because alkanes are saturated compounds where they cannot undergo addition reactions. Bromine water is also used to check the presence of aldehyde groups in the compounds. In this reaction also there is decolourisation of bromine water.
So the correct option is (D).
Note: Bromine water test works by halogenation mechanism. Bromine water test takes place at room temperature in case of gaseous alkenes but in case of liquid alkenes, the reaction will take place in presence of \[{\text{CC}}{{\text{l}}_{\text{4}}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




