
What are dipole-dipole forces?
Answer
528.6k+ views
Hint: The molecules in the compound are held by different types of intermolecular attractions and forces. One of the most important classes of intermolecular forces is dipole-dipole forces. It is possessed by molecules having permanent dipoles.
Complete answer:
Dipole-dipole forces arise due to the attraction between two dipoles. These dipole-dipole forces work the same way as the attractive forces between two oppositely charged ions. The only difference is that the charges in dipole interactions are much less.
The dipoles are simply polar molecules having partial negative and partial positive ends. Molecular dipoles occur due to the unequal dispersion of electrons between the atoms of the molecule. The more electronegative atom tends to pull the bonding pair of electrons towards itself and this results in the buildup of electron density over that atom. As a consequence of this, a dipole is formed where one region of the molecule possesses a partial negative charge and the other side a partially positive charge.
The term ‘partial charge’ is used because not all electrons get completely transferred to one atom and if the molecule is symmetrical then the dipoles get canceled and the molecule is said to be non-polar.
For example, CO2 is a nonpolar molecule while H2O is a polar molecule due to the presence of dipoles in it. Dipoles are shown with the help of an arrow where the direction of the arrow indicates the transfer of electrons from less electronegative to a high electronegative atom.

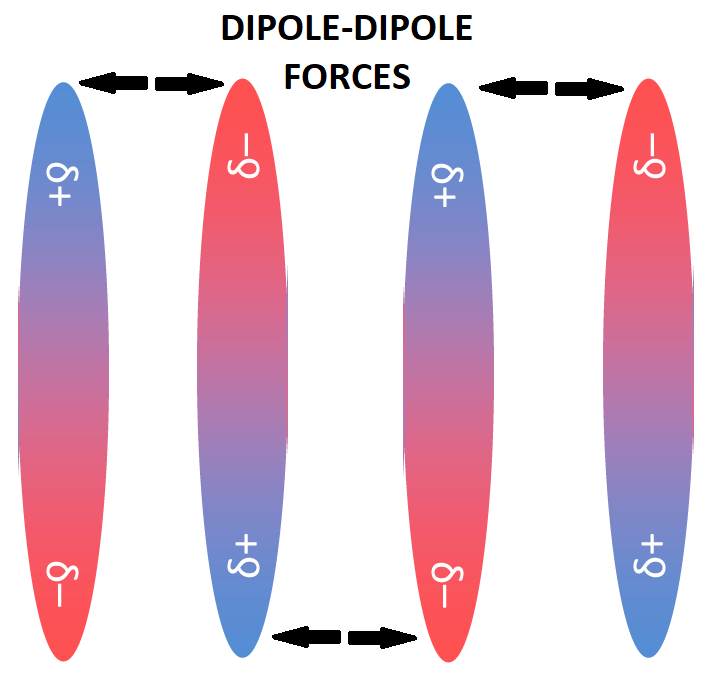
Hence, the dipole-dipole forces are the intermolecular molecular forces that arise when dipoles are very close to each other and the negative end of one dipole is attracted to the positive end of another dipole as shown in the below diagram.
Note:
The dipole-dipole forces can be either attractive or repulsive depending upon the orientation of dipoles in the compound. However, the most stable orientation is the one having a maximum number of attractive interactions.
Complete answer:
Dipole-dipole forces arise due to the attraction between two dipoles. These dipole-dipole forces work the same way as the attractive forces between two oppositely charged ions. The only difference is that the charges in dipole interactions are much less.
The dipoles are simply polar molecules having partial negative and partial positive ends. Molecular dipoles occur due to the unequal dispersion of electrons between the atoms of the molecule. The more electronegative atom tends to pull the bonding pair of electrons towards itself and this results in the buildup of electron density over that atom. As a consequence of this, a dipole is formed where one region of the molecule possesses a partial negative charge and the other side a partially positive charge.
The term ‘partial charge’ is used because not all electrons get completely transferred to one atom and if the molecule is symmetrical then the dipoles get canceled and the molecule is said to be non-polar.
For example, CO2 is a nonpolar molecule while H2O is a polar molecule due to the presence of dipoles in it. Dipoles are shown with the help of an arrow where the direction of the arrow indicates the transfer of electrons from less electronegative to a high electronegative atom.

Hence, the dipole-dipole forces are the intermolecular molecular forces that arise when dipoles are very close to each other and the negative end of one dipole is attracted to the positive end of another dipole as shown in the below diagram.

Note:
The dipole-dipole forces can be either attractive or repulsive depending upon the orientation of dipoles in the compound. However, the most stable orientation is the one having a maximum number of attractive interactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




