
What covalent compound is $ {P_4}{{\text{S}}_5} $ ?
Answer
510.6k+ views
Hint: The compound mentioned in the question $ {P_4}{{\text{S}}_5} $ is known as Tetra phosphorus Pentasulfide. It is a covalent molecule. It comes from a family of compounds known as phosphorus sulphides which contain only phosphorus and sulphur. The general formula for Phosphorus sulphide is $ {P_4}{S_x} $ with $ {\text{x}} \leqslant 10 $ .
Complete answer:
Tetra phosphorus Pentasulfide can be produced by reacting $ {{\text{P}}_4}{{\text{S}}_3} $ which is known as Phosphorus sesquisulfide with sulphur in the solution of Carbon disulphide in the presence of light amount of iodine. Iodine is used as a catalyst here.
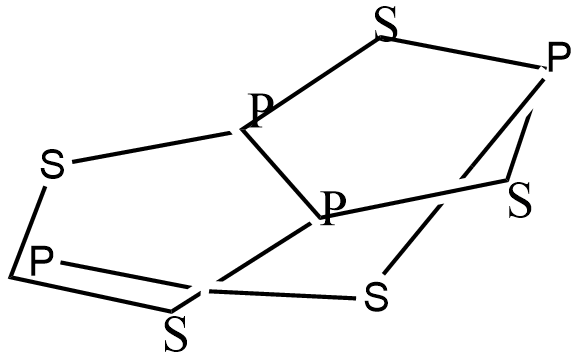
The Structure of this compound contains the arrangement of atoms and atoms are bonded to each other. all bonds are covalent bonds, tetra phosphorus Pentasulphide contains 11 non-H bonds, 11 multiple bonds, 11-aromatic bonds, 2 five-membered rings, 2 six membered rings, 2 seven-membered rings and 1 eight membered ring. The carbon atoms in tetra phosphorus Pentasulfide are located at the corners.
Tetra phosphorus Pentasulfide has a molecular mass of $ 284.2\;gmo{l^{ - 1}} $ . Tetra phosphorus Pentasulphide are appears to be a crystalline solid. it is yellow in colour it has a melting point of $ 170^\circ C $ . it has very high density of $ 2170\;Kg{m^{ - 3}} $ .Tetra phosphorus Pentasulfide is a Heterocyclic organic Compound.
Additional Information:
Phosphorous Penta sulphides have many applications. It is used as a lubricant in Zinc Dithiophosphate, it is used as a floating agent in the concentration of molybdenite minerals and it is used in production of pesticides.
Note:
Hydrogen atoms are not mentioned in the structure of tetra phosphorus Pentasulfide. To calculate hydrogen we need to remember each carbon atom is attached to sufficient hydrogen atoms to complete its covalency which is four.
Complete answer:
Tetra phosphorus Pentasulfide can be produced by reacting $ {{\text{P}}_4}{{\text{S}}_3} $ which is known as Phosphorus sesquisulfide with sulphur in the solution of Carbon disulphide in the presence of light amount of iodine. Iodine is used as a catalyst here.

The Structure of this compound contains the arrangement of atoms and atoms are bonded to each other. all bonds are covalent bonds, tetra phosphorus Pentasulphide contains 11 non-H bonds, 11 multiple bonds, 11-aromatic bonds, 2 five-membered rings, 2 six membered rings, 2 seven-membered rings and 1 eight membered ring. The carbon atoms in tetra phosphorus Pentasulfide are located at the corners.
Tetra phosphorus Pentasulfide has a molecular mass of $ 284.2\;gmo{l^{ - 1}} $ . Tetra phosphorus Pentasulphide are appears to be a crystalline solid. it is yellow in colour it has a melting point of $ 170^\circ C $ . it has very high density of $ 2170\;Kg{m^{ - 3}} $ .Tetra phosphorus Pentasulfide is a Heterocyclic organic Compound.
Additional Information:
Phosphorous Penta sulphides have many applications. It is used as a lubricant in Zinc Dithiophosphate, it is used as a floating agent in the concentration of molybdenite minerals and it is used in production of pesticides.
Note:
Hydrogen atoms are not mentioned in the structure of tetra phosphorus Pentasulfide. To calculate hydrogen we need to remember each carbon atom is attached to sufficient hydrogen atoms to complete its covalency which is four.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




