
What does induced fit mean?
Answer
492.6k+ views
Hint: As the substrate molecules are comparatively much smaller than the enzyme molecules, there should be some specific regions or sites on the enzyme for binding with the substrate. Such sites of attachment are variously called ‘active sites’ or ‘catalytic sites’ or ‘substrate sites’.
Complete Answer:
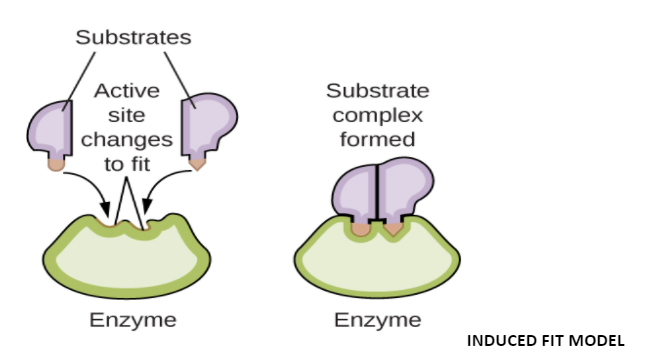
To explain the enzyme properties more efficiently, Daniel E. Koshland in 1858, modified the Fischer model. Koshland assumed that the enzyme molecule doesn’t regain its original shape and structure but the contact of the substrate induces some geometrical changes in the enzyme’s molecule active site.
Then, the enzyme molecule is made to fit completely the configuration and substrate’s active sites. At the same time, other amino acids may become more buried in the interior of the molecule. Koshland’s hypothesis has recently been confirmed by Lipscomb.
Now, discuss the Induced Fit Model.
According to this theory, the enzyme’s active site has two regions- buttressing and catalytic. The first region or buttressing region holds the substrate at a correct position while the other region or catalytic region weakens the substrate bonds by electrophilic or nucleophilic forces. When the substrate binds to the first (buttressing) region, the active site of the enzyme undergoes geometrical changes to bring the second (catalytic) region opposite to substrate bonds and this initiates the reaction.
Note:
In this the hydrophobic and charged groups both are involved in substrate binding. A phosphoserine and the –SH group of cysteine residue are involved in catalysis. Other amino acid residues that are not involved in either substrate binding or catalysis are lysine and methionine.
Complete Answer:
To explain the enzyme properties more efficiently, Daniel E. Koshland in 1858, modified the Fischer model. Koshland assumed that the enzyme molecule doesn’t regain its original shape and structure but the contact of the substrate induces some geometrical changes in the enzyme’s molecule active site.
Then, the enzyme molecule is made to fit completely the configuration and substrate’s active sites. At the same time, other amino acids may become more buried in the interior of the molecule. Koshland’s hypothesis has recently been confirmed by Lipscomb.
Now, discuss the Induced Fit Model.
According to this theory, the enzyme’s active site has two regions- buttressing and catalytic. The first region or buttressing region holds the substrate at a correct position while the other region or catalytic region weakens the substrate bonds by electrophilic or nucleophilic forces. When the substrate binds to the first (buttressing) region, the active site of the enzyme undergoes geometrical changes to bring the second (catalytic) region opposite to substrate bonds and this initiates the reaction.

Note:
In this the hydrophobic and charged groups both are involved in substrate binding. A phosphoserine and the –SH group of cysteine residue are involved in catalysis. Other amino acid residues that are not involved in either substrate binding or catalysis are lysine and methionine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




