
What is a benzene ring?
Answer
492.9k+ views
Hint: Benzene is one of the most important organic compounds with the chemical formula \[{C_6}{H_6}\]. Benzene is the parent compound of the various aromatic compounds. Benzene is a naturally occurring compound that is found in many plants and animals and is produced by volcanoes and forest fires, but it is also a major industrial chemical made from coal and oil.
Complete Step By Step Answer:
The hexagonal configuration observed in Benzene, an organic chemical molecule, is known as the Benzene ring. The benzene ring has a hexagonal structure with six carbon atoms connected by single or double bonds. Each carbon atom in the ring is connected to a hydrogen atom. The carbon atom may be connected to a single atom or a group of atoms in various situations.
The hexagonal configuration can be seen in benzene and its derivatives molecules. In the petroleum business, it is a natural component of crude oil. It is a crucial component of gasoline because of its high-octane number.
Because its molecules include only carbon and hydrogen, benzene is categorised as a hydrocarbon.
Properties of benzene are as follows:
Benzene is immiscible in water but soluble in organic solvents.
It is a colourless liquid with a pleasant smell.
It weighs $0.87$ gram per cubic metre. It has a lower density than water.
Benzene has a high melting point and a moderate boiling point.
The structure of benzene is as follows:
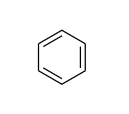
Note:
Acute benzene exposure is harmful to the central nervous system; however, the myelotoxic and potential chromosomal damaging and leukemogenic effects of benzene must be considered when evaluating the chronic effects.
Complete Step By Step Answer:
The hexagonal configuration observed in Benzene, an organic chemical molecule, is known as the Benzene ring. The benzene ring has a hexagonal structure with six carbon atoms connected by single or double bonds. Each carbon atom in the ring is connected to a hydrogen atom. The carbon atom may be connected to a single atom or a group of atoms in various situations.
The hexagonal configuration can be seen in benzene and its derivatives molecules. In the petroleum business, it is a natural component of crude oil. It is a crucial component of gasoline because of its high-octane number.
Because its molecules include only carbon and hydrogen, benzene is categorised as a hydrocarbon.
Properties of benzene are as follows:
Benzene is immiscible in water but soluble in organic solvents.
It is a colourless liquid with a pleasant smell.
It weighs $0.87$ gram per cubic metre. It has a lower density than water.
Benzene has a high melting point and a moderate boiling point.
The structure of benzene is as follows:

Note:
Acute benzene exposure is harmful to the central nervous system; however, the myelotoxic and potential chromosomal damaging and leukemogenic effects of benzene must be considered when evaluating the chronic effects.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




