
What is a protective colloid?
Answer
609k+ views
Hint: We should recall the nature of lyophilic colloids against lyophobic colloids. Presence of lyophilic colloids is beneficial for lyophobic colloids.
Complete step by step answer:
We should know that a protective colloid is a lyophilic colloid that when present in small quantities keeps lyophobic colloids from precipitating under the coagulating action of electrolytes. We should know that lyophilic sols are more stable than lyophobic sols. It is due to the fact that lyophilic colloids are extensively solvated that means colloidal particles are covered by a sheath of the liquid in which they are dispersed.
It is interesting to know that lyophilic colloids have a unique property of protecting lyophobic colloids. When a lyophilic sol is added to the lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect the latter from electrolytes. Lyophilic colloids used for this purpose are called protective colloids.
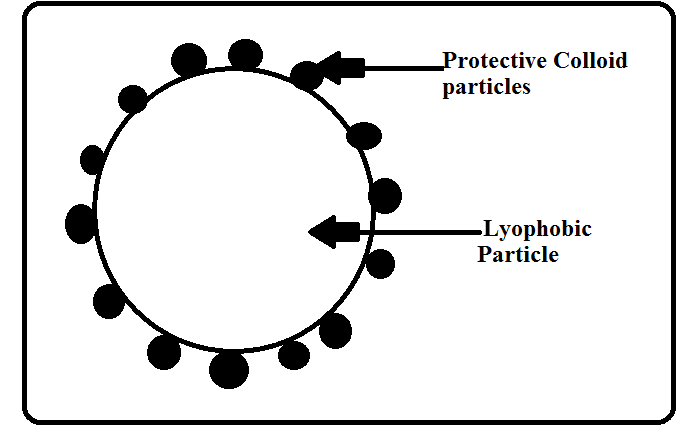
Some examples of protective colloids that we should remember are mentioned below along with their gold number. Knowledge of the gold number is important.
We should know that Gold Number is the number of milligrams of a protective colloid which prevents the coagulation of 10 ml of a standard hydro gold sol, on coagulation changes from red to blue, which is prevented by a protective colloid. Coagulation of gold sol is indicated by colour change from red to blue/purple when particle size just increases.
We should know that more is the gold number; less is the protective power of the lyophilic colloid since it means that the amount required is more. The amount is taken in terms of weight in milligrams.
Starch (gold no. =25)
Gum Arabic (gold no.=0.15)
Egg albumin (gold no.=0.08)
Gelatin (gold no.=0.005)
Note: We should know about lyophilic colloids. They are solvent loving colloids. In lyophilic colloids, the dispersed phase shows a positive affinity for the dispersion medium (solvent). The high affinity of dispersed particles with the dispersed medium is due to the formation of a large number of hydrogen bonds. Example: Starch or protein dissolved in water in water. Preparation of lyophilic colloids is much easier by mixing the particle in the dispersion medium.
Whereas, lyophobic colloids are solvent hating colloids. In lyophobic colloids, the dispersed phase does not have any attraction for the dispersion medium. . Example: Ferric hydroxide or Aluminium hydroxide dissolved. The preparation of lyophobic colloids is not direct, special methods are required.
Complete step by step answer:
We should know that a protective colloid is a lyophilic colloid that when present in small quantities keeps lyophobic colloids from precipitating under the coagulating action of electrolytes. We should know that lyophilic sols are more stable than lyophobic sols. It is due to the fact that lyophilic colloids are extensively solvated that means colloidal particles are covered by a sheath of the liquid in which they are dispersed.
It is interesting to know that lyophilic colloids have a unique property of protecting lyophobic colloids. When a lyophilic sol is added to the lyophobic sol, the lyophilic particles form a layer around lyophobic particles and thus protect the latter from electrolytes. Lyophilic colloids used for this purpose are called protective colloids.

Some examples of protective colloids that we should remember are mentioned below along with their gold number. Knowledge of the gold number is important.
We should know that Gold Number is the number of milligrams of a protective colloid which prevents the coagulation of 10 ml of a standard hydro gold sol, on coagulation changes from red to blue, which is prevented by a protective colloid. Coagulation of gold sol is indicated by colour change from red to blue/purple when particle size just increases.
We should know that more is the gold number; less is the protective power of the lyophilic colloid since it means that the amount required is more. The amount is taken in terms of weight in milligrams.
Starch (gold no. =25)
Gum Arabic (gold no.=0.15)
Egg albumin (gold no.=0.08)
Gelatin (gold no.=0.005)
Note: We should know about lyophilic colloids. They are solvent loving colloids. In lyophilic colloids, the dispersed phase shows a positive affinity for the dispersion medium (solvent). The high affinity of dispersed particles with the dispersed medium is due to the formation of a large number of hydrogen bonds. Example: Starch or protein dissolved in water in water. Preparation of lyophilic colloids is much easier by mixing the particle in the dispersion medium.
Whereas, lyophobic colloids are solvent hating colloids. In lyophobic colloids, the dispersed phase does not have any attraction for the dispersion medium. . Example: Ferric hydroxide or Aluminium hydroxide dissolved. The preparation of lyophobic colloids is not direct, special methods are required.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




