
What is bad ozone and good ozone?
Answer
535.5k+ views
Hint: ozone is an inorganic gas molecule, having chemical formula $ O_3 $. It is pale blue in color with a pungent smell. Structurally, it is a bent molecule with $ C_{2V} $ symmetry. It is a polar molecule having a dipole moment of $ 0.53D $ .
Complete answer:
Ozone is a gas that can be produced in two ways:
Naturally, it is formed in the upper layer of the atmosphere of our planet (from oxygen). It protects us from most of the sun’s harmful ultraviolet radiation (UV) radiations.
In the lower atmosphere, ozone can be produced as a result of human activities and this form is toxic for our health.
GOOD OZONE- The ozone layer that is present in the upper atmosphere is protective in nature and thus it is vital to life on earth. This ozone is referred to as the good ozone. It helps in shielding us from the harmful UV radiations of the sun. UV rays are potential carcinogens, which can cause skin cancer and even destroy plants. There are certain harmful chemicals that have the ability to destroy upper ozone, such as Freon refrigerants.Therefore, strict guidelines need to be taken and followed in order to conserve and restore ozone layer.
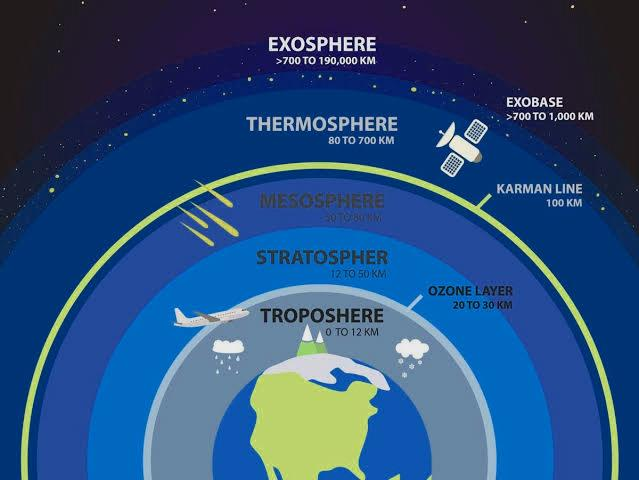
BAD OZONE- Smog is a distinctive form of urban air pollution. It is usually referred to as bad ozone. Ozone has been categorized as a secondary pollutant by the scientists, because it is produced by the combination of precursor pollutants - emanating from factories, cars, power or chemical plants -along with volatile organic compounds, and the sunlight.
The formation of ground-level ozone pollutant can be written in the following chemical equations-
$ NO_2 + h\nu + O_2 \to NO + O_3 $
$ R + 2NO + 2O_2 \to RO + 2NO_2 + H_2O $
Note:
Ozone is extremely harmful for our health, especially for our respiratory system. It can damage our lungs, and cause death in severe cases. Even an exposure for a short period of time can lead to shortness of breath by reducing lung function.
Complete answer:
Ozone is a gas that can be produced in two ways:
Naturally, it is formed in the upper layer of the atmosphere of our planet (from oxygen). It protects us from most of the sun’s harmful ultraviolet radiation (UV) radiations.
In the lower atmosphere, ozone can be produced as a result of human activities and this form is toxic for our health.
GOOD OZONE- The ozone layer that is present in the upper atmosphere is protective in nature and thus it is vital to life on earth. This ozone is referred to as the good ozone. It helps in shielding us from the harmful UV radiations of the sun. UV rays are potential carcinogens, which can cause skin cancer and even destroy plants. There are certain harmful chemicals that have the ability to destroy upper ozone, such as Freon refrigerants.Therefore, strict guidelines need to be taken and followed in order to conserve and restore ozone layer.

BAD OZONE- Smog is a distinctive form of urban air pollution. It is usually referred to as bad ozone. Ozone has been categorized as a secondary pollutant by the scientists, because it is produced by the combination of precursor pollutants - emanating from factories, cars, power or chemical plants -along with volatile organic compounds, and the sunlight.
The formation of ground-level ozone pollutant can be written in the following chemical equations-
$ NO_2 + h\nu + O_2 \to NO + O_3 $
$ R + 2NO + 2O_2 \to RO + 2NO_2 + H_2O $
Note:
Ozone is extremely harmful for our health, especially for our respiratory system. It can damage our lungs, and cause death in severe cases. Even an exposure for a short period of time can lead to shortness of breath by reducing lung function.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




