
What is electron configuration?
Answer
524.1k+ views
Hint: The electron configuration of any element describes how the electrons will be distributed in its atomic orbitals. the configuration follows the standard notation while distributing the electrons in a proper sequence.
Complete answer:
Let us understand the basics of electron configuration;
Electron configuration is in short, the summary of electrons around the nucleus. The electrons are filled up into the sequential orbitals in a dignified sequence. The order of electrons which are placed in those shells depends on the order of energy they carry. This is known as Aufbau’s principle; as stated below,
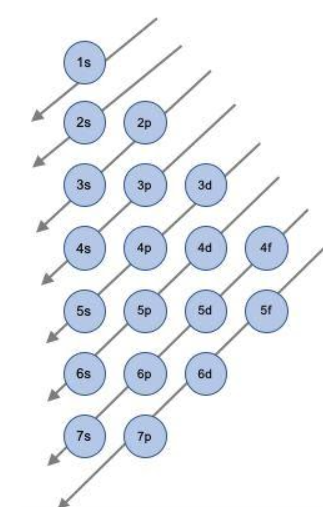
This is the sequence in which the electrons are being filled in the shells around the nucleus. For example;
$_{11}Na=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$
Sometimes, the condensed notation is used instead of standard notation i.e. $_{10}Ne=\left[ He \right]2{{s}^{2}}2{{p}^{6}}$ .
The above-described configuration denotes the energy level, type of shell/orbital along with number of electrons as-
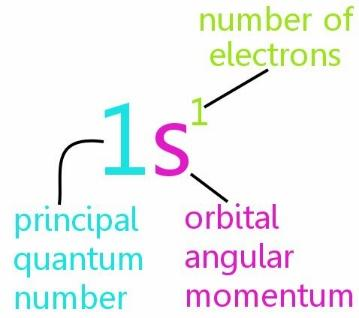
The electron configuration is used to determine the valency of an element, interpret atomic spectra and predict the properties of similar types of elements.
Note:
Do note that there are exceptions to the electron configuration discussed above. The two main exceptions are shown by chromium and copper. In these cases, completely or half-filled subshells are more stable than that of partially filled d-orbital. So, an electron is excited from 4s subshell towards a 3d orbital.
Complete answer:
Let us understand the basics of electron configuration;
Electron configuration is in short, the summary of electrons around the nucleus. The electrons are filled up into the sequential orbitals in a dignified sequence. The order of electrons which are placed in those shells depends on the order of energy they carry. This is known as Aufbau’s principle; as stated below,

This is the sequence in which the electrons are being filled in the shells around the nucleus. For example;
$_{11}Na=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$
Sometimes, the condensed notation is used instead of standard notation i.e. $_{10}Ne=\left[ He \right]2{{s}^{2}}2{{p}^{6}}$ .
The above-described configuration denotes the energy level, type of shell/orbital along with number of electrons as-

The electron configuration is used to determine the valency of an element, interpret atomic spectra and predict the properties of similar types of elements.
Note:
Do note that there are exceptions to the electron configuration discussed above. The two main exceptions are shown by chromium and copper. In these cases, completely or half-filled subshells are more stable than that of partially filled d-orbital. So, an electron is excited from 4s subshell towards a 3d orbital.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




