
What is entropy? Write its units.
Answer
578.7k+ views
Hint: This is thermodynamic function. Generally, it is used to express the extent of disorder in a system.
Complete step by step answer:
Entropy is a measure of randomness or disorder of a system. It is usually represented by ‘S’.
Let us explain entropy by giving example of states of matter i.e. gas, liquid and solid
As we know molecular arrangement of solid, liquid and gas are as follows
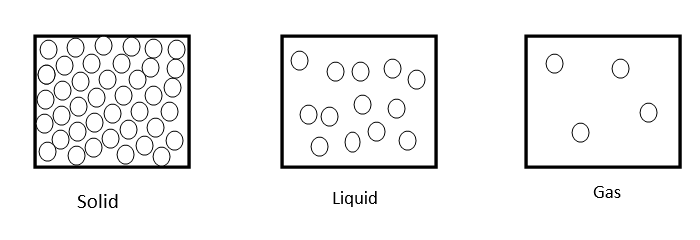
Molecular attraction in solid is greatest, in liquids it is moderate and in gaseous state it is least.
$\therefore $ Randomness of molecules in gas, liquid, solid decrease as
gas > liquid > Solid.
Since randomness in molecules of gas is greater due to least attraction and decreases from liquid and then solid due to increased attraction between molecules.
$\therefore $ Entropy of states of matter in the order
Gas > liquid > solid.
Entropy is a state function and entropy change during a process given by $\Delta S = {S_2} - {S_1}$
Where${S_2} = \sum\limits_{}^{} {{S_{product}}} $${S_1} = \sum\limits_{}^{} {{S_{reac\tan t}}} $
Change in entropy of system changes as follows
(i) When the system absorbs energy in the form of heat, the kinetic energy of the system increases. As a result disorder vibration of molecules increases.
$\therefore $ entropy of the system also increases.
(ii) Entropy is inversely related to temperature
$\Delta S = \dfrac{{{q_{rev}}}}{T}$
Where, ${q_{rev}} = $ heat absorbed in reversible process.
$T = $ Absolute temperature.
For same amount of heat at low temperature entropy i.e. randomness is more
$\therefore \Delta S$ is inversely proportional to absolute temp $T$
Unit of entropy is $Cal$${k^{ - 1}}$ $mo{l^{ - 1}}$ in C.G.S system and $J$${K^{ - 1}}$ $mo{l^{ - 1}}$ in S.I. system.
Note:
Entropy is extensive property. Entropy increases when solid melts as sublimes or decomposes to give gas or liquid. Entropy also increases when the number of molecules of a product is greater than the number of molecules of Reactant.
Complete step by step answer:
Entropy is a measure of randomness or disorder of a system. It is usually represented by ‘S’.
Let us explain entropy by giving example of states of matter i.e. gas, liquid and solid
As we know molecular arrangement of solid, liquid and gas are as follows

Molecular attraction in solid is greatest, in liquids it is moderate and in gaseous state it is least.
$\therefore $ Randomness of molecules in gas, liquid, solid decrease as
gas > liquid > Solid.
Since randomness in molecules of gas is greater due to least attraction and decreases from liquid and then solid due to increased attraction between molecules.
$\therefore $ Entropy of states of matter in the order
Gas > liquid > solid.
Entropy is a state function and entropy change during a process given by $\Delta S = {S_2} - {S_1}$
Where${S_2} = \sum\limits_{}^{} {{S_{product}}} $${S_1} = \sum\limits_{}^{} {{S_{reac\tan t}}} $
Change in entropy of system changes as follows
(i) When the system absorbs energy in the form of heat, the kinetic energy of the system increases. As a result disorder vibration of molecules increases.
$\therefore $ entropy of the system also increases.
(ii) Entropy is inversely related to temperature
$\Delta S = \dfrac{{{q_{rev}}}}{T}$
Where, ${q_{rev}} = $ heat absorbed in reversible process.
$T = $ Absolute temperature.
For same amount of heat at low temperature entropy i.e. randomness is more
$\therefore \Delta S$ is inversely proportional to absolute temp $T$
Unit of entropy is $Cal$${k^{ - 1}}$ $mo{l^{ - 1}}$ in C.G.S system and $J$${K^{ - 1}}$ $mo{l^{ - 1}}$ in S.I. system.
Note:
Entropy is extensive property. Entropy increases when solid melts as sublimes or decomposes to give gas or liquid. Entropy also increases when the number of molecules of a product is greater than the number of molecules of Reactant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




