
What is geminal dihalide?
Answer
526.1k+ views
Hint: The description for the “Geminal” word refers to the relationship between two atoms or functional groups attached to the same carbon atom. The word geminal comes from the Latin word Gemini meaning “twins”.
Complete step by step answer: Dihalide is the di-halogen derivative of a halogen-containing organic compound. Halogen containing organic compounds are those which contain an equal number of halogen replaced by one or more than hydrogen atoms in either aliphatic or aromatic hydrocarbons.
Geminal dihalides are those dihalides in which the same halogen atom is present on the same carbon atom.
For example:
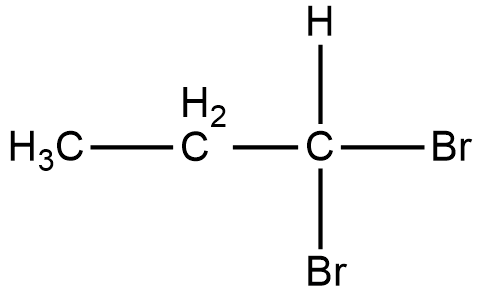
Geminal dihalides are also known as geminal dihalides. In the common system, they are named as alkylidene dihalides. And the positions on the same carbon atom are called germinal positions.
Geminal dihalides are prepared by the addition reaction of vinyl halide. When vinyl halide undergoes additional reaction with hydrogen chloride then the formation of geminal dihalide takes place.
In the IUPAC system, they are named as di-haloalkane in which the position or the locant for the halogen, after being repeated twice, is prefixed to the name of the di-haloalkane.
Additional Information: If the compound has the same halogens on adjacent carbons, then they are known as vicinal dihalides. They are prepared by a reaction between a halogen and an alkene. In our language, we can say vicinal dihalides and geminal dihalides are the brothers from two different mothers.
Note: The concept of geminal is important in the branch of chemistry including synthesis and spectroscopy. Since the functional group often shows different behavior when they are connected to the same atom and when they are connected to a different atom.
Complete step by step answer: Dihalide is the di-halogen derivative of a halogen-containing organic compound. Halogen containing organic compounds are those which contain an equal number of halogen replaced by one or more than hydrogen atoms in either aliphatic or aromatic hydrocarbons.
Geminal dihalides are those dihalides in which the same halogen atom is present on the same carbon atom.
For example:

Geminal dihalides are also known as geminal dihalides. In the common system, they are named as alkylidene dihalides. And the positions on the same carbon atom are called germinal positions.
Geminal dihalides are prepared by the addition reaction of vinyl halide. When vinyl halide undergoes additional reaction with hydrogen chloride then the formation of geminal dihalide takes place.
In the IUPAC system, they are named as di-haloalkane in which the position or the locant for the halogen, after being repeated twice, is prefixed to the name of the di-haloalkane.
Additional Information: If the compound has the same halogens on adjacent carbons, then they are known as vicinal dihalides. They are prepared by a reaction between a halogen and an alkene. In our language, we can say vicinal dihalides and geminal dihalides are the brothers from two different mothers.
Note: The concept of geminal is important in the branch of chemistry including synthesis and spectroscopy. Since the functional group often shows different behavior when they are connected to the same atom and when they are connected to a different atom.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




